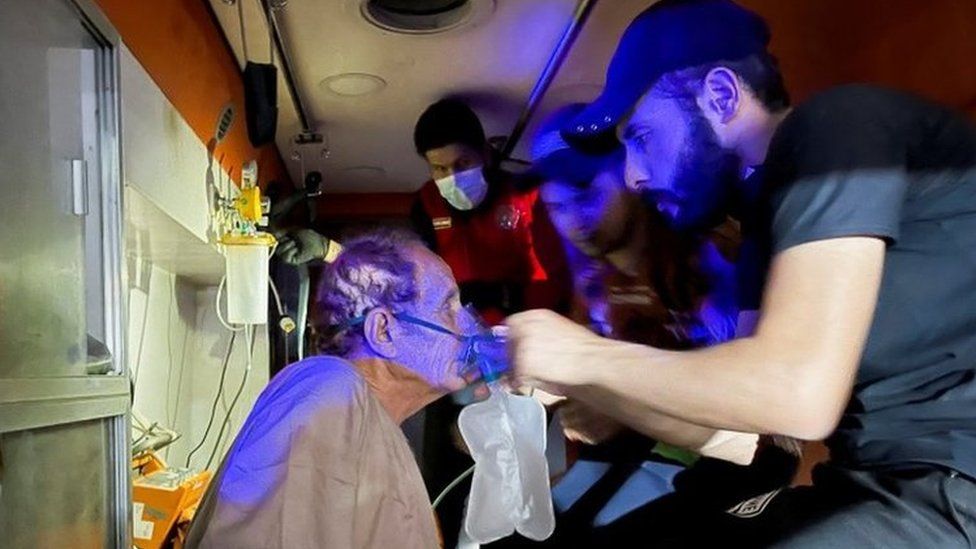
U.S. experts weigh the risks for younger women and cases of a rare blood-clotting disorder, and lift the pause in giving the one-shot vaccine.Use of the one-shot Johnson & Johnson Covid vaccine will resume within days, but with a warning added to its label about the risk for a rare blood-clotting disorder that has occurred among young women, the Food and Drug Administration said on Friday.Concerns about the disorder had led to a pause in the use of the vaccine that began 10 days earlier. The F.D.A. decided against limiting the vaccine’s use by age or gender, although some European countries have imposed such restrictions on a vaccine made by AstraZeneca because of a similar clotting disorder. Federal health officials said information about the disorder would be provided at vaccination sites. Dr. Peter Marks, the F.D.A.’s top vaccine regulator, predicted that the vaccine could be deployed again by Saturday morning.The Johnson & Johnson suspension came after officials learned that six women had developed a severe disorder that led to blood clots in their brains within about two weeks of receiving the vaccine. One died. The pause was widely considered a blow to national and global vaccination efforts and removed an effective vaccine that many states and countries had counted on to deploy in hard-to-reach places. Unlike the vaccines made by Pfizer-BioNTech and Moderna, Johnson & Johnson’s vaccine requires only one shot and is easier to store and distribute because it does not require extremely cold temperatures. At the advisory panel meeting about the blood-clotting issue on Friday, the C.D.C. reported that it had identified a total of 15 cases, including three deaths. But the risk is considered extremely small — nearly eight million Americans have received the Johnson & Johnson vaccine — and the panel ultimately decided that the vaccine’s benefits far outweighed its risks. It also concluded that failing to use it would lead to more deaths than the clotting disorder might cause.The panel voted 10 to 4 to resume using the vaccine with the warning label. Some who voted “no” wanted a more prominent warning about the risk to younger women and the availability of other Covid vaccines that do not appear to pose the same risk. Lifting the pause will allow many states to restart giving the one-dose shot to college students and hard-to-reach populations like rural Americans, migrants and the homebound elderly. Their access had been hampered by the suspension, which left roughly 10 million doses sitting on shelves.In Wisconsin, where one-third of the population is fully vaccinated, officials said they intended to begin using their Johnson & Johnson doses as soon as possible. “We have also heard from a number of vaccinators who say that there are lots of people who don’t like needles,” said Julie Willems Van Dijk, the deputy secretary of the state health department. “They just want to get the vaccine that requires them to have one shot versus two shots.”Dr. Rochelle P. Walensky, the C.D.C. director, said governors had expressed intense interest in resuming use of the shot.“They wondered why we had paused and they were anxious to have this back, have an opportunity for a single-dose vaccine, for a one-and-done possibility,” she said.About 135.8 million people in the United States have received at least one shot of a coronavirus vaccine since the rollout began. But since last week, average daily doses have fallen by almost 13 percent, from a peak of 3.38 million daily doses, on average, to about 2.95 million, on average. It is the first time since early April that providers have given fewer than three million shots a day, on average.It is difficult to say exactly what is driving the decrease. Even before the use of the Johnson & Johnson vaccine was paused, shipments of it had dipped because of a factory error that had ruined millions of doses.Larry Bergner, the administrator for the health department in Newton County, Mo., population 58,000, said demand had been falling before the pause. He was concerned that the federal government’s decision had made hesitancy about the vaccine in the area worse.“Some tell me that they had planned on getting vaccinated until J.&J. was halted,” Mr. Bergner said. “Now, they say they are going to hold off until they feel confident that all vaccines are safe.”The county had distributed fliers and made phone calls to businesses, churches and other community groups to drum up interest for a vaccination clinic on Wednesday, but only 14 people showed up. It was actually about double what Mr. Bergner had expected, he said, though the health department could have handled about 100 shots.Dr. Walensky said the federal government planned to emphasize the vaccine’s safety to Americans who might be hesitant to take it after the pause.“We have to do extraordinary outreach to clinicians, as we have been doing this past week,” she said. “We already have plans to start that on Monday, to public health officials. And then we have to do extraordinary outreach to patients, to meet people where they’re at, to educate them.”She said the C.D.C. had talked to health providers for young women, including the American College of Obstetricians and Gynecologists. The pause and investigation into the rare disorder, she added, should give the public confidence in the system used to monitor vaccine safety.Registration for the Johnson & Johnson vaccine at the Charlotte Maxeke Johannesburg Academic Hospital in South Africa last month. Shots will resume there next week, officials announced Thursday.João Silva/The New York TimesEuropean Union regulators earlier this week did not recommend the kind of age restrictions for the Johnson & Johnson shots that some countries have imposed on the AstraZeneca vaccine. Johnson & Johnson did agree to include a warning of risks for the blood clots. Several European countries have since resumed use of the Johnson & Johnson vaccine, as has South Africa. Up until just hours before the pause was recommended by American officials, regulators had planned for a revision to the F.D.A.’s emergency use authorization similar to the one formalized Friday, with warnings about the blood clots. But top health officials decided in a meeting on April 12 that the government should call for a pause while federal authorities and the C.D.C.’s expert panel investigated a possible link between the clots and the vaccine. They feared a number of cases of the disorder had not been identified and wanted to allow more time for those who had just received the vaccine to reach the point at which the rare clotting typically appears. “As we did this intensive scientific evaluation over recent days, I think we became more and more confident about the decision that was made today,” Dr. Janet Woodcock, the acting F.D.A. commissioner, said on Friday.In the C.D.C. panel analysis, women between 30 and 39 appear to be at greatest risk, with 11.8 cases per million doses given. Among women 18 to 49, there have been seven cases per million doses. The condition, which the C.D.C. is calling thrombosis with thrombocytopenic syndrome, causes severe blood clots and also a tendency to bleed at the same time, because of abnormally low levels of platelets, a blood component involved in clotting.The disorder is “rare but clinically serious,” Dr. Tom Shimabukuro, the deputy director of the C.D.C.’s immunization safety office, said at the meeting.Additional potential cases, including some in men, are being reviewed. There was also a case in a 25-year-old man who participated in a clinical trial of the vaccine.The patients’ symptoms closely resemble a rare syndrome that can be caused by heparin, a widely used blood thinner, Dr. Michael Streiff, a hematologist at Johns Hopkins University, told the panel. Heparin, typically used to treat blood clots, should not be given to these patients, he said.Symptoms include severe headaches, abdominal pain, leg pain or shortness of breath. Those problems generally do not set in before about six days after the vaccination. Once the symptoms occur, treatment should begin as soon as possible, because it can worsen rapidly, researchers say. Dr. Marks, the F.D.A. regulator, said the agency was recommending blood thinners other than heparin, and a blood product called intravenous immune globulin, which can help ease the immune reaction causing the problem.“That appears to reverse this process,” he said.Officials warned that women under 50 have an increased risk of a rare but serious blood-clotting disorder, and “may choose” to receive a different vaccine instead.Mario Tama/Getty ImagesResearchers suspect that in these rare cases, the vaccine causes an intense reaction by the patient’s immune system, which churns out antibodies that activate platelets, a blood component needed for clotting. Why this occurs in some people, many of them younger women, is not known, and experts say that they have so far been unable to identify traits or underlying conditions that may make some people susceptible. Top U.S. health officials have stressed that finding the small number of cases of a rare disorder, and pausing use of the vaccine, indicated that safeguards were in place to assess risks and to raise awareness among doctors and hospitals about the unusual symptoms.“This pause was essential to our ability to inform the public,” Dr. José R. Romero, chairman of the expert panel, the Advisory Committee on Immunization Practices, said on Friday.But some public health experts have expressed concerns that the government’s actions would fuel vaccine hesitancy just when the Biden administration is heavily pushing to have all adults immunized by the summer.The impact of the Johnson & Johnson vaccine on vaccine hesitancy is still evolving. A poll released this week from Ipsos/Axios found that the pause itself boosted confidence in federal vaccine monitors, with 81 percent saying that the C.D.C. and the F.D.A. acted appropriately. The sentiment was unusually bipartisan, with 87 percent of Republicans and 91 percent of Democrats sharing that view.Measuring the impact of the Johnson & Johnson pause is rather tricky because as Liz Hamel, vice president of public opinion and research survey at the Kaiser Family Foundation, said, “You don’t know what the trajectory of the vaccine uptake would have been in the absence of this pause.”The biggest challenge ahead, she noted, will be the creation of vaccine-confidence messages that resonate, especially in light of the setback with Johnson & Johnson. “We don’t know whether it has increased hesitancy among women in particular,” she said.Rob Barss, a paramedic, talked with Romeo Porier before administering a Johnson & Johnson shot, part of a mobile vaccine distribution program in Leominster, Mass.Cj Gunther/EPA, via ShutterstockBenjamin Mueller, Matina Stevis-Gridneff, Julie Bosman, Jan Hoffman, Carl Zimmer and Emily Anthes contributed reporting.
Read more →