Why I Gave My Mosaic Embryo a Chance
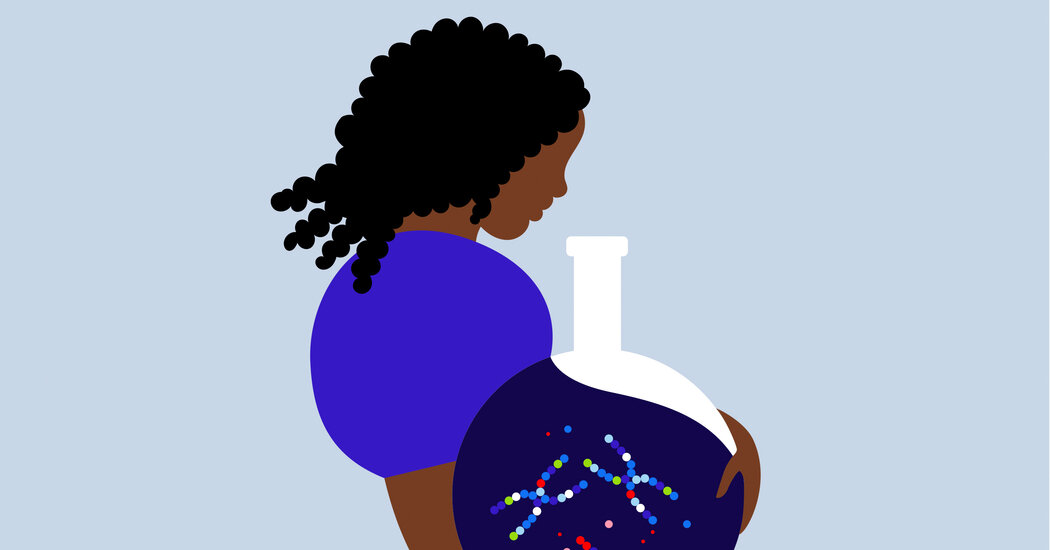
‘It was like rolling the dice, except for someone you’ve never met.’My husband and I were sitting in an Upper East Side office with deep-toned velvet couches and fluffy throw pillows, surrounded by photos of smiling babies, as the fertility doctor gave his spiel. He told us that after age 35, a woman’s chances of getting pregnant drop. Older women produce few normal embryos even with fertility treatment. But we’d have a healthy baby in our arms within a year — if we tested the embryos.By testing the chromosomes in my embryos, he said, we could weed out the abnormal embryos that may lead to miscarriage or a child with disabilities and only use viable ones.I’ve always been a late bloomer — I met my husband at 37 and married at 39. I was in good health but pushing 40, with diminishing egg count and quality. After six months of trying to conceive on our own, we wanted all the help we could get. My husband and I jumped at the embryo testing suggestion.After two long rounds of in vitro fertilization, we had five embryos, but the genetic testing deemed four of them “abnormal,” meaning they contained extra or missing chromosomes. Our fifth embryo, a girl, was what our genetic counselor called “mosaic,” meaning it had both abnormal and normal cells.Starting in the late 1990s, doctors testing fertilized eggs classified them as normal or abnormal, then added the classification “mosaic” in 2015. Mosaic embryos can be either low- or high-level, depending on the number of abnormal cells. Twenty percent of tested embryos are mosaic.Ours was a low-level mosaic embryo, with a few cells having an extra 22nd chromosome. Scientists are still trying to understand mosaicism, but this meant our embryo could be normal and lead to a healthy baby; she could have genetic abnormalities that would lead to miscarriage; or she could be born with congenital heart defects, asymmetrical development (meaning one side of her body could look like it was melting while the opposite side looked normal) or other disabilities that would cause her to use a wheelchair for life. It was like rolling the dice, except for someone you’ve never met.It turns out there are a lot of online communities for mosaic kids and their families, including one on Facebook dedicated specifically to mosaics with an extra 22nd chromosome. Some adults lived normal lives and only find they have mosaic +22 later in life. Some women who were pregnant with babies with mosaic +22 miscarried. Children — ranging from newborns to young adults — had varying developmental challenges.What scared me most was that in girls, the extra 22nd chromosome could cause infertility. I felt selfish for wanting her so desperately that I would allow her into the world without this same opportunity.We had to make a fast choice: do a third cycle of I.V.F., hoping to get a normal embryo, or risk transferring the mosaic. Should we first try the mosaic embryo or risk having more nonviable embryos to agonize over? Because of the risks to the fetus and the developmental challenges our baby might face, the genetic counselor advised us to not transfer.I had always hoped my future children wouldn’t be short like me. My husband, who sprouts freckles in the sun, hoped they would inherit my darker skin. Otherwise, we had no lofty dreams of them going to Harvard or making any “world’s most beautiful baby” list. We picked a dog that was the runt of the litter, with a lopsided face, because we thought she was modern art. But that’s a lot different from bringing a child into the world knowing it had a risk of living a difficult life.It was a lot to take in. I wasn’t scared that my life would be curtailed if I brought up a child with special needs — I was ready to dedicate myself to a child. But I worried that my wanting a child was blinding me to some of my potential shortcomings. Was I capable of giving up everything to concentrate on this person who would need me in ways I couldn’t even fathom yet? I was terrified that I couldn’t handle having a child with special needs and would take it out on her.I was also a little embarrassed that I cared so much about having a “perfect” baby that fit the standard 46-chromosome human body. Who was I to make this life and death decision for another human?But it turns out that I didn’t know as much as I thought I did. Because genetic tests of I.V.F. embryos are far from perfect.“Labs only test five cells from around 150 that make up the fertilized egg,” said Dr. Hugh Taylor, chairman of the Department of Obstetrics, Gynecology and Reproductive Sciences at the Yale School of Medicine. “We’re fooling ourselves if we think we have full information on an embryo based on those few cells.”A recently published study of 1,000 mosaic embryos found those that progressed into a late-term pregnancy and full term birth had similar odds of being born without any discernible genetic differences to a normal embryo. But there were no guarantees.I didn’t want to try another I.V.F. cycle. In late February 2020, we decided to transfer the embryo into my uterus — just in time for New York City to shut down during the pandemic.Five months later, I got a call from a physician who was filling in for my doctor; she canceled my appointment, claiming she was uncomfortable transferring a mosaic embryo. I was livid and overcome with grief.“The larger question that emerges with embryo testing is who gets to take on the risk of possibly bringing a child with potential disabilities into the world,” Dr. Taylor said. “The decision should not be left to physicians. Patients should be given the freedom to decide, and properly counseled in cases where there are abnormalities that will inevitably lead to death.”Parents I had met online described wheeling or driving their frozen abnormal and mosaic embryos in unwieldy metal tanks to other clinics when their physicians refused to transfer. Fortunately, my regular doctor came back and scheduled a new appointment for the following month.My husband and I got lucky. Our beautiful, imperfect embryo attached to the uterine wall, mesmerizing us with her wild beating heart at biweekly ultrasounds. As each week brought on fresh worries — that I could miscarry, that the baby might have other abnormalities not caught at embryo testing — I found comfort in Dr. Taylor’s words: “Mosaicism is more common than we think. Many of us are mosaic without knowing it.”At three months, my doctor recommended a blood test that checked the baby’s DNA fragments in my blood to see if she was at risk for genetic abnormalities. At this point, my husband and I had begun to notice families in the dog park whose children had genetic disabilities. We quietly found acceptance that we would add variety to the families in our community and decided that we wouldn’t terminate the baby — no matter the result.They came back as normal. But like embryo testing, the blood test couldn’t diagnose a fetus’s genetic condition with certainty. Our doctor offered a more accurate amniocentesis test, but we had already made our decision. I decided to leave it there.Now, during ultrasounds, our daughter hides her face behind her hands or presses hard against the placenta, as if asking us to let her grow in privacy. The last time I glimpsed her full profile, at five months gestation, her nose, long and sharp, was prominent and unmistakable. I wondered if it was one of the characteristics of the extra 22nd chromosome or if she’d simply inherited my husband’s nose. As my due date draws nearer, her genetic profile is less of a concern. I’m thrilled we’ve made it this far.Jacquelynn Kerubo is a writer and public health communicator.
Read more →