Author: Pam Belluck
Barnard College Plans to Offer Abortion Pills on Campus
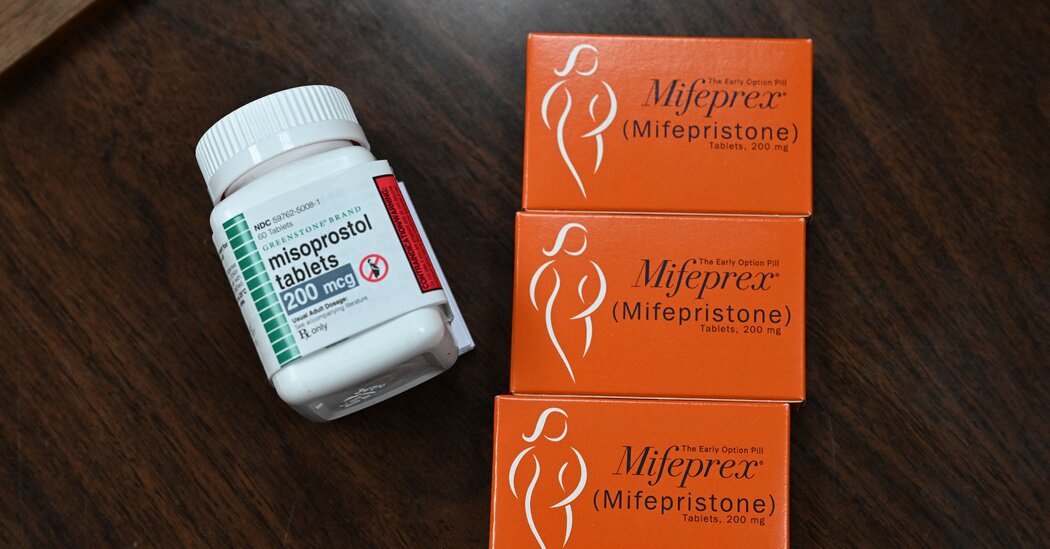
It is one of many colleges and universities deliberating over how to respond to the fall of Roe v. Wade.Barnard, the private women’s college in New York City, will begin offering abortion pills on campus next year, college officials announced Thursday.The decision, to take effect in September, signals how the nation’s colleges and universities are becoming another front in the nation’s pitched battle over abortion after the Supreme Court decision to overturn Roe v. Wade.“I think we’re putting a stake in the ground that we believe that health and wellness is really the institution’s responsibility for students, and we want to do everything we can to support our students,” Sian L. Beilock, the president of Barnard, which operates in partnership with Columbia University, said in an interview.In an email sent out to the campus Thursday, Barnard officials wrote, “The overturning of Roe v. Wade after 50 years will likely decrease college accessibility, result in lower graduation rates, and derail employment trajectories.” The email said that while abortion access in New York was currently strong, “we are also preparing in the event that there is a barrier to access in the future, for any reason.”This summer, Massachusetts enacted a law requiring the state’s public colleges and universities to submit plans by November 2023 to provide abortion pills to students, either through their campus health centers or through a local service that is readily accessible to students. By January, California’s public colleges and universities will be required by a state law enacted in 2019 to offer the pills.At the same time, colleges in states with abortion bans or strict restrictions are imposing measures to rein in campus reproductive health services.Last month, the general counsel of the University of Idaho sent a memo to all employees saying that under Idaho’s near-total ban on abortion, which took effect in August, employees of the state university cannot counsel patients about abortion or refer them to abortion services. If employees do so, the memo said, they could be charged with a misdemeanor or a felony under the new law, lose their job and be permanently barred from state employment.The memo also said that the Idaho law prohibited employees from dispensing emergency contraception, except in cases of rape, and added that, because it was unclear how broadly the law applied to other methods of contraception, “we are advising a conservative approach here, that the university not provide standard birth control itself.”Condoms can be provided only if they are intended to stop the spread of sexually transmitted diseases, the memo said, “not for purposes of birth control.”Dr. Marina Catallozzi, Barnard’s vice president of health and wellness and chief health officer. “We feel like this is of course a natural step in caring for a population of college students who are at risk of pregnancy,” she said.Barnard CollegeSian L. Beilock, Barnard’s president. “We believe that health and wellness is really the institution’s responsibility for students,” she said.Barnard CollegeIn general, colleges and universities have cautiously approached the question of whether to offer medication abortion. Rachel Mack, a spokeswoman for the American College Health Association, which represents more than 700 institutions of higher education, said the issue was being discussed among colleges and the association’s reproductive rights task force.“Not all schools have the resources to provide such a service and may refer out to community providers,” Ms. Mack said. “Campuses vary greatly in their available resources — this can be due to location, the needs of their student population and many other factors.”A few colleges decided to offer abortion pills long before this year’s Supreme Court decision overturning Roe. The University of California, Berkeley, has been doing so for a couple of years. A study published in 2018 estimated that 322 to 519 students at California’s public universities sought medication abortion each month and that many faced obstacles of cost, of travel distance to providers and of long waits for appointments.In a survey by the American College Health Association in 2020, 2.5 percent of 122 college health centers said they provided medication abortion on campus, while 87 percent said they provided referrals to patients seeking abortion.At Barnard, Dr. Marina Catallozzi, the vice president of health and wellness and chief health officer, said that adding medication abortion was already on the college’s radar before some students met with health services officials this year to ask the school to provide it.“We feel like this is of course a natural step in caring for a population of college students who are at risk of pregnancy,” Dr. Catallozzi said, noting that Barnard already offered a range of reproductive health services, including a campus vending machine for emergency contraception for students who want to obtain the morning-after pill in a more private way than visiting the wellness center.“With every reproductive health decision, but particularly around a pregnancy,” she said, “we want to make sure that students have all of the options — if they want to continue a pregnancy, if they want to continue and go on to adoption, if they want to terminate.”The Wellness Spot at Barnard College, a health hub that offers services, workshops and other assistance to students.Barnard CollegeBarnard officials said they decided not to offer the pills immediately in order to spend the next several months training staff, developing protocols and working out logistics. They said that the pills would be covered by the college’s health insurance plan and that there would be emergency funding available for students without insurance or those who do not want to use their parents’ insurance policies.Medication abortion, legalized in the United States in 2000, typically involves two drugs: mifepristone, which blocks a hormone necessary for pregnancy development, followed 24 to 48 hours later by misoprostol, which causes contractions that expel pregnancy tissue. The Food and Drug Administration requires that mifepristone be dispensed by specially certified providers, but patients can take the pills on their own at home or any location they choose.Patients with some medical issues, like bleeding disorders, are not prescribed abortion pills. But for the many patients who are medically eligible, data indicates medication abortion is safe and effective, with a small percentage of patients requiring a procedure to fully remove pregnancy tissue and an even smaller proportion experiencing serious complications.Dr. Catallozzi and Dr. Beilock said very few students at Barnard had sought abortions in recent years. Those who do are typically referred to services affiliated with Columbia University Irving Medical Center, and that relationship will continue. Dr. Catallozzi said she expected that some students who live in dorms may prefer to go to the medical center for surgical abortions, which do not involve the days of bleeding that typically follow taking the pills.Dr. Catallozzi also said that the college wanted to offer the option in case New York’s abortion services become overwhelmed with patients from states with restrictions. “It makes sense for us to say, ‘Hey, the landscape’s changing all over the country. Let’s make sure that if needed, we’re prepared,’” she said.In the 2023 academic year, Dr. Beilock will become the president of Dartmouth College. Asked if she would recommend that Dartmouth offer abortion pills, Dr. Beilock said: “I’m looking forward to getting to know the Dartmouth community better. Right now, I’m president of Barnard and thinking, with our experts, about what’s best for Barnard.”
Read more →Price of New A.L.S. Treatment Will Be $158,000 Per Year, Maker Says
The list price of the therapy, Relyvrio, is much higher than an economic research group recommends, but the company says most patients will pay very little themselves.A new medication for A.L.S., the devastating neurological disorder that causes paralysis and death, will have a list price of $158,000 a year, its manufacturer disclosed Friday.The treatment, to be marketed as Relyvrio, is a combination of two existing drugs and will be available to patients in the United States in about four to six weeks, according to officials of the company, Amylyx Pharmaceuticals.Relyvrio was approved by the Food and Drug Administration on Thursday, even though the agency’s analysis concluded there was not yet sufficient evidence that the medication could help patients live longer or slow the rate at which they lose functions like muscle control, speaking or breathing without assistance.The F.D.A. decided to greenlight the drug instead of waiting until 2024 for results of a large clinical trial partly because the treatment is considered to be safe. The agency said that although the evidence of effectiveness was uncertain, “given the serious and life-threatening nature of A.L.S. and the substantial unmet need, this level of uncertainty is acceptable in this instance.”A.L.S., or amyotrophic lateral sclerosis — also called Lou Gehrig’s disease — often strikes patients in the prime of life and frequently causes death within two to five years. It is diagnosed in about 6,000 people worldwide each year, and Amylyx estimates that there are about 29,000 people living with the disease in the United States.The Fight Against A.L.S.The illness, also called Lou Gehrig’s disease, robs people of their ability to move, speak, eat and ultimately breathe.Relyvrio: The experimental treatment for A.L.S. conceived a decade ago by two college students received the Food and Drug Administration’s approval, despite questions about its effectiveness.A Runner’s Mission: After surpassing the average life expectancy for people with the disease, Andrea Peet decided to race a new kind of clock: 50 marathons in 50 states.Brain Implant: A man who is fully paralyzed by A.L.S. was able to communicate using only his thoughts.Rethinking Care: In 2017, Brian Wallach was diagnosed with A.L.S. Now, his startup aims to help other patients make the most of their time.Amylyx officials predicted that most patients would pay little or nothing for the treatment because the company expects insurers, both private and public, to cover it. Amylyx plans to provide it free to uninsured patients experiencing financial hardship.Still, the list price is much higher than that recommended by the Institute for Clinical and Economic Review, a nonprofit organization that evaluates the value of medicines. In a statement, the group’s chief medical officer, Dr. David Rind, said that while “there are clear benefits to patients with a rapidly fatal disease to have early access to a safe therapy,” his organization had concluded that “an annual price of $9,100 to $30,700 would be reasonable if the therapy actually works.”Dr. Rind added that “while awaiting proof, we believe that patients would benefit from a price closer to the price of production of Relyvrio rather than a price more than five times higher than the top of a value-based range.”During an investor conference call on Friday, Justin Klee, a founder of Amylyx, said the price was chosen after meeting with insurers, patients, doctors and others. He said the company considered what would allow it to “invest in new treatments so that A.L.S. first becomes a manageable chronic condition and ultimately is cured,” and added that the price “allows Amylyx to sustain programs to help people who can benefit from Relyvrio access it.”The F.D.A. has approved only two other A.L.S. medications. Riluzole, a tablet approved in 1995, can extend survival by several months and generally costs significantly less than $10,000 a year. Edaravone, marketed as Radicava, can slow symptom progression by about 33 percent. Radicava, which was originally approved in 2017 as an intravenous infusion, was approved this year in an oral form that carries a list price of $171,000 a year. Amylyx officials said they expected that, as in the company’s clinical trials, many patients would take Relyvrio, a powder that is mixed with water, along with one or both of the other medications.Relyvrio was conceived by the founders of Amylyx, Mr. Klee and Joshua Cohen, when they were undergraduate students at Brown University less than a decade ago. They proposed that combining taurursodiol, an over-the-counter supplement sometimes used to regulate liver enzymes, and sodium phenylbutyrate, a prescription medication for a pediatric urea disorder, could protect neurons in the brain from damage in diseases like A.L.S. by preventing dysfunction of two structures in cells: mitochondria and the endoplasmic reticulum.In an interview, Mr. Klee said the company expected that private insurers would cover the drug with no co-payments for patients, and he said that Amylyx would work to make it affordable for people on Medicare or Medicaid and provide it free to those without insurance who are financially struggling. Mr. Klee noted that patients had been obtaining the ingredients on their own for some time, buying the taurursodiol supplement from Amazon and paying up to $11,000 a month for the sodium phenylbutyrate.“Now that our product is approved, we have to be laser-focused on making sure that people can access it,” Mr. Klee said.Relyvrio’s clinical trials included patients who developed symptoms of A.L.S. within 18 months before the trial and were affected in at least three body regions, which is generally a sign of fast-progressing disease. The F.D.A.’s approval did not restrict which patients could use the medication. Mr. Cohen said in an interview that Amylyx didn’t have projections of how many would.“There are patients who are just receiving the diagnosis today, and there are patients who are making end-of-life decisions today, and I think that people in different circumstances are going to make very different treatment decisions,” he said.Amylyx has also conducted a small trial of Relyvrio in Alzheimer’s patients, and Mr. Cohen discussed plans to test it for other neurodevelopmental disorders.“We conceived the drug to try to target pathways of neuronal death,” he said, “and neuronal death is an important part of not just A.L.S. but potentially many diseases.”
Read more →FDA Approves ALS Treatment Despite Questions About Effectiveness
The drug is safe, and one trial found it may extend survival and slow paralysis in functions like muscle control and speaking. A larger trial will be completed in 2024.The Food and Drug Administration on Thursday approved an experimental treatment for A.L.S., a severe neurological disorder that causes paralysis and death, despite questions about the therapy’s effectiveness.The treatment, conceived about a decade ago by two college students, was approved even though analyses by the F.D.A.’s reviewers concluded there was not yet sufficient evidence that the medication could help patients live longer or slow the rate at which they lose functions like muscle control, speaking or breathing without assistance. Nevertheless, the agency decided to greenlight the drug without waiting two years for results of a large clinical trial, citing data showing the treatment to be safe and the desperation of patients with a disease that often causes death within two-to-five years.The drug, which has the scientific name AMX0035, will be marketed as Relyvrio. In a summary memorandum about the drug the F.D.A. wrote that there was “residual uncertainty about the evidence of effectiveness,” but that “given the serious and life-threatening nature of A.L.S. and the substantial unmet need, this level of uncertainty is acceptable in this instance.” The memorandum also said that the benefits outweigh the risks because the treatment is “without any significant safety signals of concern.”The approval follows an impassioned campaign by patients and advocacy groups. In addition, doctors who treat A.L.S. patients had urged approval in a letter to the F.D.A. and testimony and in testimony before an F.D.A. advisory committee.“In your difficult job, there’s always going to be a chance of making a mistake; it comes down to which mistake you would rather make,” Dr. Richard Bedlack, director of the A.L.S. clinic at Duke University, testified this month. “To approve AMX0035 and find out in two years that it doesn’t work — I doubt many are going to be very angry because people with A.L.S. got to try something that was safe and appeared promising in 2022.”But, he added, “Can you imagine the mistake of saying no and then getting confirmatory evidence in two years that this really did work? And realizing all those patients were much more disabled or even dead when they didn’t need to be? I don’t know how you’ll be able to live with yourself if you make that mistake.”A.L.S., or amyotrophic lateral sclerosis, also called Lou Gehrig’s disease, is diagnosed in about 6,000 people worldwide each year. There are only two other approved A.L.S. medications in the United States: riluzole, approved in 1995, which can extend survival by several months, and edaravone, approved in 2017, which can slow progression by about 33 percent.The Fight Against A.L.S.The illness, also called Lou Gehrig’s disease, robs people of their ability to move, speak, eat and ultimately breathe.Relyvrio: The experimental treatment for A.L.S. conceived a decade ago by two college students received the Food and Drug Administration’s approval, despite questions about its effectiveness.A Runner’s Mission: After surpassing the average life expectancy for people with the disease, Andrea Peet decided to race a new kind of clock: 50 marathons in 50 states.Brain Implant: A man who is fully paralyzed by A.L.S. was able to communicate using only his thoughts.Rethinking Care: In 2017, Brian Wallach was diagnosed with A.L.S. Now, his startup aims to help other patients make the most of their time.Relyvrio was conceived by Justin Klee and Joshua Cohen when they were undergraduate students at Brown University. They proposed that combining taurursodiol, a supplement sometimes used to regulate liver enzymes, and sodium phenylbutyrate, a medication for a pediatric urea disorder, could protect neurons in the brain from damage in diseases like A.L.S. by preventing dysfunction of two structures in cells: mitochondria and the endoplasmic reticulum. They later founded a small Massachusetts company, Amylyx Pharmaceuticals.As patients learned about the compound, some began obtaining the ingredients on their own from Amazon and other sources. In June, Canada became the first country to approve the treatment, under a special condition requiring Amylyx to later provide better evidence that it worked. Advocacy groups predicted that some American patients would seek it from Canada, where it is marketed as Albrioza, if the F.D.A. didn’t approve it.The two-drug combination was conceived by Justin Klee, left, and Joshua Cohen when they were undergraduates at Brown University.Cody O’Loughlin for The New York TimesAmylyx did not immediately say what price it is considering for the treatment in the United States and has said the price is still being negotiated in Canada. “Amylyx’s goal is that every person who is eligible for Relyvrio will have access as quickly and efficiently as possible as we know people with A.L.S. and their families have no time to wait,” the company said in a statement after the F.D.A. announcement.The medication, a bitter-tasting powder mixed with water and either drunk or ingested through a feeding tube, traveled an unusual and controversial path to approval. The F.D.A. typically requires two persuasive clinical trials, usually Phase 3 trials, which are larger and more extensive than Phase 2 studies. For serious diseases with few treatments, the agency can accept one trial plus additional confirmatory data.For Relyvrio, the data comes only from one Phase 2 trial in which 137 patients took either the drug or a placebo, plus an extension study that followed some patients after the trial ended when they were knowingly taking the drug.The Phase 2 trial involved patients considered to have fast-progressing disease. Two-thirds of participants received Relyvrio. Over 24 weeks, they experienced a 25 percent slower decline than participants receiving placebo — declining 2.32 points less on a 48-point A.L.S. scale that rates 12 physical abilities, including walking, speaking, swallowing, dressing, handwriting and breathing.The open-label extension study involved 90 of those patients, including 34 from the placebo group, who began taking the medication about seven months after those who received it from the beginning. Patients who received the treatment the longest had a median of about 6.5 months more time before being hospitalized, being put on a ventilator or dying, Amylyx reported. Researchers later published another analysis that suggested additional benefit.The F.D.A. initially recommended that Amylyx not apply for approval until the Phase 3 trial was completed in 2024.A.L.S. advocacy groups campaigned vehemently to persuade the F.D.A. to reconsider, especially after the agency’s controversial approval last year of the Alzheimer’s drug Aduhelm despite doubts about whether it worked. Soon after, F.D.A. officials began suggesting that Amylyx submit an application for approval using existing data.In March, a committee of independent advisers to the F.D.A. voted by a narrow margin that the treatment had not yet been shown to be effective, a conclusion also reached by the F.D.A.’s own reviewers. The agency then allowed Amylyx to submit more data and took the unusual step of scheduling a second independent advisory committee meeting on Sept. 7. In a report presented there, agency reviewers said they also considered the new data insufficient.But Dr. Billy Dunn, director of the F.D.A.’s office of neuroscience, told the advisory committee that “although some might reasonably argue that substantial evidence does not currently exist” to justify approval, the agency should exercise “the broadest flexibility” by also considering the seriousness of the disease and the dearth of available treatments.Dr. Dunn posed a question to the company: If the treatment received approval now and was shown to be ineffective in the Phase 3 trial, would Amylyx voluntarily withdraw it from the market, saving the agency a lengthy recall process? Mr. Klee said the company would.That commitment by Amylyx, plus emotional testimony from patients and doctors, persuaded more advisory committee members at the September meeting, where the vote favoring approval was seven to two.One of those who voted against approval, Dr. Kenneth Fischbeck, a distinguished investigator for the National Institutes of Health, said the company could provide the therapy to patients for free while waiting for better evidence, but “I don’t think it’s met the standard of evidence to allow them to sell the drug.” Mark Weston, a member of the advisory committee who has A.L.S., said he was disappointed that the new information the company presented wasn’t stronger. “I was hoping for something more,” he said. But Mr. Weston, who earlier in the meeting named A.L.S. patients who had died since the March hearing, said he voted for approval because “I can’t decouple my thoughts about that from my thoughts about the unmet need.”That need for treatments was the overwhelming message of patients who testified. “Instead of thinking you are protecting me I want you to recommend approval so that I have the chance to live,” said Brian Wallach, 41, who co-founded “I AM ALS,” an advocacy group, in testimony mostly read by a friend because A.L.S. has profoundly damaged his ability to speak.Gregory Canter, one of the clinical trial participants, said “the rate of my functional decline has slowed considerably” since beginning the drug over three years ago.“A.L.S. is the basement; this starts us up the stairs,” Mr. Canter said. “It won’t get us to the top alone, but each step up is important to point us in the right direction.”Calaneet Balas, president and chief executive of the A.L.S. Association, said in a statement that the F.D.A. decision was “a victory for the entire ALS community, which came together to advocate for early approval of AMX0035.” The association and other advocacy groups met with F.D.A. officials, submitted a petition with over 50,000 signatures and organized a campaign generating over 13,000 emails to the agency.The A.L.S. Association contributed $2.2 million to the development and study of AMX0035, using money raised through the 2014 Ice Bucket Challenge. Amylyx agreed to use sales of the drug to repay 150 percent of the association’s grant to fund more research.
Read more →Post-Roe Decision, Abortion Pill Providers Work to Broaden Access
As bans and restrictions proliferate across the country, abortion pill providers are pushing the envelope of regulations and laws to meet the surging demand for medication abortion in post-Roe America.Some are using physician discretion to prescribe pills to patients further along in pregnancy than the 10-week limit set by the Food and Drug Administration. Some are making pills available to women who are not pregnant but feel they could need them someday. Some are employing a don’t-ask-don’t-tell approach, providing telemedicine consultations and prescriptions without verifying that patients are in states that permit abortion.These changes are easing access to the pills for patients in states that have curtailed abortion, and also in states where it remains legal, but where clinics have longer wait times as patients flood in from restrictive states.Some of the practices, like not confirming that telemedicine patients are located in states that allow abortion, may run afoul of anti-abortion state laws or fall into uncharted legal territory, but they may also be challenging to police, reproductive health experts said.“We’re going to see these different approaches by organizations as they assess what the laws say and develop their rationale for how to provide care,” said Elizabeth Nash, state policy analyst for the Guttmacher Institute, a research group supporting abortion rights. “We just don’t have a road map about how to provide medication abortion post-Roe, so it’s all being created right now.”Abortion opponents criticized the efforts, calling them risky for women. Tessa Longbons, senior research associate at the Charlotte Lozier Institute, an anti-abortion organization, said the method had greater rates of complications and failure after 10 weeks that “could just put women at risk.” Providing pills to women who are not pregnant, she said, creates a risk that a woman “could take it anytime or someone that it wasn’t initially prescribed to could end up taking it.”Medication abortion, which was legalized in the United States in 2000, typically involves two drugs: mifepristone, which blocks a hormone necessary for pregnancy development, followed 24 to 48 hours later by misoprostol, which causes contractions that expel pregnancy tissue.Patients with some medical issues, like bleeding disorders, are not prescribed abortion pills. But for the many patients who are medically eligible, data indicates medication abortion is safe and effective, with a small percentage of patients requiring a procedure to fully remove pregnancy tissue and an even smaller proportion experiencing serious complications.The method is less expensive, less invasive and, especially with telemedicine, more private than surgical abortions. By 2020, it accounted for over half of U.S. abortions and has become even more sought-after since Roe was overturned.In the fast-changing abortion landscape, new online medication-abortion services are starting, and existing services are expanding, often adopting some of the new practices. Legally, this is a new frontier, both supporters and opponents of abortion rights say. It’s unclear, for example, whether any anti-abortion state laws — which typically target providers, not patients — address providing pills to someone who is not pregnant.Read More on Abortion Issues in AmericaSounding the Alarm: A United Nations panel reviewing racial issues in the United States called on the Biden administration to take action to protect abortion access for minorities and low-income people, adding that these groups could be disproportionately hit by the end of Roe.In Michigan: A state board in Michigan refused to place an abortion rights referendum on the November ballot because of a dispute over word spacing on the petition, which included more than 750,000 signatures.Fetal Personhood: A push to grant fetuses the same legal rights as people is gaining momentum, as anti-abortion activists move beyond bans and aim to get the procedure classified as murder.“As these new practices are developing, it is likely that they will be in a legal gray area,” said John Seago, president of Texas Right to Life, who said current Texas laws only involve providing abortion to women known to be pregnant.Other practices, like those that help patients in states with bans obtain pills, might violate those laws. But questions remain. Most providers using these practices prescribe and distribute pills only within states where abortion is legal. If those legally prescribed pills are then used by residents of anti-abortion states, it’s unclear if those states can prosecute out-of-state providers.Dr. Seago, who has a doctorate in bioethics, said new legislation would probably be introduced to combat the new practices. “Legislators are committed to figuring out how to enforce these laws,” he said.Beyond the F.D.A. ThresholdIn the fast-changing abortion landscape, new online medication-abortion services are starting and existing services are expanding.Jeff Roberson/Associated PressBefore Roe was overturned, few American services offered pills beyond the F.D.A.’s 10-week threshold, but many are now.Abortion Telemedicine, which was started after a draft of the Supreme Court ruling overturning Roe leaked in May, serves patients throughout the first trimester, which is 13 weeks into pregnancy.That company, and others serving patients at 11 or 12 weeks’ gestation, can legally use medical discretion to do so because studies suggest that abortion pills are safe and effective at that stage. The World Health Organization supports medication abortion through 12 weeks’ gestation.Dr. Daniel Grossman, a professor of obstetrics, gynecology and reproductive sciences at the University of California, San Francisco, said that late in the first trimester, medication abortion is safe and effective, but that there’s “a somewhat higher risk of some complications, including heavy bleeding,” and an additional dose of misoprostol is often needed to fully expel the tissue.Some services, including Abortion Telemedicine, automatically send a second round of the four misoprostol tablets for patients undergoing late first-trimester abortions.Reproductive health experts said patients should be advised that, while earlier in pregnancy, expelled tissue resembles a heavy period, after 10 weeks, it can appear more fetus-like. Dr. Abigail R.A. Aiken, an associate professor at the University of Texas, Austin, who leads a medication-abortion research group, said preparing patients for what the tissue might look likecould also help them guard against legal risk in states banning abortion — for example, in a situation where a patient is surprised by what they see and “then is disclosing that to someone who’s like ‘Well, I’m going to report you.’”Joann, 23, a single mother, was already 10 weeks pregnant when she decided to abort, so she contacted Abortion Telemedicine. She said she initially planned to carry her pregnancy to term, but then her 3-year-old son was diagnosed with autism and her employer, the U.S. military, decided to transfer her to another state. Joann, who asked to be identified only by her first name to protect her privacy, was in Colorado at the time, where abortion is legal, but her community was conservative.The service’s nurse practitioner told her that since she’d be taking the pills after 10 weeks’ gestation, she should expect more pain and bleeding, and counseled that the expelled tissue might resemble a fetus “so that I would be prepared for it,” Joann said.At 11 weeks and two days pregnant, she took the mifepristone, followed by the first four-tablet dose of misoprostol the next day and the second round six hours later. The cramping hurt, but “it was bearable,” she said.Providing Pills Before PregnancyHana is not pregnant but sought abortion pills just in case she did have an unwanted pregnancy in a state with a strict abortion ban, a practice called advance provision.Adriana Zehbrauskas for The New York TimesSome services are offering “advance provision” of abortion pills to patients who aren’t pregnant. Christie Pitney, a midwife who works with Aid Access, a medication-abortion service based in Europe that works with U.S. providers, likened it to travelers who “get medication for traveler’s diarrhea or for altitude sickness before you actually need to use it.” She said patients completed medical consultations before receiving prescriptions and were asked to contact the service again for an evaluation before taking the pills. Mifepristone’s shelf life is three to five years and misoprostol’s is 18 to 24 months, experts say.Several reproductive health experts have recently endorsed advance provision, suggesting that safety risks are low if patients are medically screened before being prescribed pills and before taking them, and if they have access to medical care if needed. Still, providers acknowledge that in an environment where many states restrict abortion, some patients may not follow appropriate guidelines. An article supporting advance provision, co-written by Dr. Grossman at U.C.S.F., noted that patients could take the pills in inappropriate circumstances or give them to others, and recommended further study.Ms. Pitney said Aid Access, which began offering advance provision in the United States in September 2021, was receiving about 40 requests per day earlier this year, but that the week after the Supreme Court decision, over 10,000 requests poured in.Hana, 31, said she sought advance provision because she lives in Arizona, a conservative state where a ban or other restrictions could take effect, and which is one of 19 states prohibiting telemedicine abortion. Hana, a claims researcher for a health insurer, who asked to be identified by only her first name to protect her privacy, said she tried to order from Aid Access the day the Supreme Court overturned Roe, but couldn’t get onto the website because traffic was so heavy. Two days later, she succeeded.After patients complete medical consultation forms, Aid Access ships from within the United States to patients in states with legal telemedicine abortion, while patients in restrictive states, like Hana’s, receive pills from a pharmacy in India. In 2019, the F.D.A. tried unsuccessfully to get Aid Access to halt overseas shipping. The organization’s founder, Dr. Rebecca Gomperts, a Dutch physician, said that U.S. Customs had occasionally stopped packages, but that most arrived without incident.“I have it hidden in my closet,” said Hana, who lives alone, but doesn’t want guests to stumble upon the medication. “I’m a little nervous,” she said, but added, “It’s really nice to have it just in case.”An Honor System for Patients’ LocationAbortion medication in the storage bin of a shuttered abortion clinic in Texas.Evelyn Hockstein/ReutersSome services check IP addresses to ensure that during telemedicine-abortion consultations patients are located in states where the practice is legal, but a growing number have decided not to verify location.“We don’t have any barriers such as an ID verification or GPS validation,” Dr. Jayaram Brindala, the founder of Abortion Telemedicine, said. His company arranges video consultations in 17 states where telemedicine abortion is legal and its appointment-booking form asks patients where they live, but “it’s on their honor,” he said.Some patients indicate they reside in states with abortion restrictions and are traveling to states where abortion is legal to receive pills at an address there, where the company can legally ship them, Dr. Brindala said.Ms. Pitney, the midwife with Aid Access, who also runs a service based in the United States called Forward Midwifery, said a shipping address was the only geographical information those services required. Only if patients mention they are doing the medical consultation from a state that prohibits telemedicine abortion or shipping of pills will she say she cannot legally treat them and refer them to Plan C, an online clearinghouse for information about medication abortion.Plan C recently added information about “virtual mailboxes” with commercial mail-forwarding companies: addresses in states where pills can legally be shipped and forwarded to patients in restrictive states. Forwarding companies are most likely unaware of the contents of the nondescript packages.“You’re using a legal mailing service and you’re using a legal telehealth service and you’re getting F.D.A.-approved products from a clinician with a prescription and the providers are fully compliant with the rules,” said Elisa Wells, co-founder and co-director of Plan C, which tested mail-forwarding companies by shipping bottles filled with garbanzo beans to approximate the rattling of pill bottles.Some medication-abortion providers are trying to broaden access while following F.D.A. guidelines and verifying patients’ locations.Abortion on Demand, which serves patients only up to nine weeks’ gestation, checks that patients do their video consultations from one of 22 states or Washington, D.C., all locations where telemedicine abortion is legal, and it will not ship to mail-forwarding companies or P.O. Boxes.“We very explicitly say that we’re sort of banning anything that may not just put us in legal jeopardy, but really put patients and put patients’ friends who are helping them in legal jeopardy,” Leah Coplon, director of clinical operations, said.Still, she said, her organization recently expanded to Pennsylvania. That state allows telemedicine abortion, but since Abortion on Demand has no office in Pennsylvania, state law prevents it from shipping pills there. Instead, Pennsylvania patients pick up pills at FedEx sites in New Jersey or Maryland.“We’ve had a lot of interest,” she said. “If the Pennsylvania model works well, there may be the potential to expand it in other areas.”
Read more →Abortion Pills Take the Spotlight as States Impose Abortion Bans
Demand for medication abortion is surging, setting the stage for new legal battles.In the hours after the Supreme Court released its decision overturning the legal right to abortion in the United States, nearly 100 requests for appointments flowed into Just the Pill, a nonprofit organization that arranges for patients to obtain abortion pills in several states.That was about four times the usual daily number of appointment requests for the organization, and many came from patients in Texas and other states that quickly halted abortions after the court ruling.Abortion pills, already used in more than half of recent abortions in the U.S., are becoming even more sought-after in the aftermath of Roe v. Wade being overturned, and they will likely be at the center of the legal battles that are expected to unfold as about half the states ban abortion and others take steps to increase access.The method, known as medication abortion, is authorized by the Food and Drug Administration for use in the first 10 weeks of pregnancy. It involves taking two different drugs, 24 to 48 hours apart, to stop the development of a pregnancy and then to cause contractions similar to a miscarriage to expel the fetus, a process that usually causes bleeding similar to a heavy period.Many patients choose medication abortion because it is less expensive, less invasive and affords more privacy than surgical abortions — the pills can be received by mail and taken at home, or anywhere, after an initial consultation with a doctor by video, phone, in person or even just by filling out an online form.The patient must participate in the consultation from a state that allows abortion, even if it simply involves being on the phone in a car just over the border. The IP address of the computer or phone they use allows the clinic to identify where they are.For states that ban all forms of abortion, medication abortion is likely to provide significant enforcement challenges. It is one thing to shut down a clinic; it is much harder to police activities like sending or receiving pills through the mail or traveling to a state where pills are legal to have a consultation and pick them up, legal experts say.“When people say we’re going back to the days before Roe, there’s no such thing as a time machine — we have a very different pharmaceutical landscape,” said Katie Watson, a constitutional scholar and medical ethicist at the Feinberg School of Medicine at Northwestern University.The abortion laws beginning to take effect in numerous conservative states ban all forms of abortion, including medication abortion. In addition, 19 states already had laws barring using telemedicine for abortion. Texas recently enacted a law prohibiting sending abortion pills through the mail. So groups and some state governments that support abortion rights are mobilizing to help patients obtain the pills in states where they are legal.Since October 2020, Just the Pill has provided more than 2,500 telemedicine consultations with doctors to supply abortion pills by mail to patients in Colorado, Minnesota, Montana and Wyoming. Within a few days, it plans to deploy in Colorado the first of what will become “a fleet of mobile clinics” to park along state borders, providing consultations for medication abortions and dispensing pills, said Dr. Julie Amaon, the organization’s medical director.Called “Abortion Delivered,” the clinic-on-wheels program, which will also provide surgical abortions for patients who prefer it or are too far along in pregnancy for a medication abortion, is designed to reach patients from nearby states like Texas, Oklahoma and South Dakota that quickly outlawed abortion after the court decision, as well as other states like Utah that are expected to ban or sharply restrict abortion.“By operating on state borders, we will reduce travel burdens for patients in states with bans or severe limits,” Dr. Amaon said. “And by moving beyond a traditional brick-and-mortar clinic, our mobile clinics can quickly adapt to the courts, state legislatures, and the markets, going wherever the need is.”Similar medication abortion providers are also planning for an influx. Hey Jane, an organization that has served nearly 10,000 patients in California, Colorado, Illinois, New Mexico, New York and Washington, plans to expand to more states. “We’ve ramped up our team to accommodate this significant increase in demand,” said its chief executive, Kiki Freedman.Anti-abortion groups are trying to counter the rise in interest in medication abortion by claiming it is unsafe, calling it “chemical abortion.” James Studnicki, vice president of data analytics at Charlotte Lozier Institute, an arm of Susan B. Anthony Pro-Life America, said on Friday that “the safety of the abortion pill is greatly exaggerated,” and called the rise in medication abortion “a serious public health threat.”Much remains unknown about how states that ban all or most abortions will try to enforce their laws in cases of medication abortion. But as the Biden administration scrambled to react to the court ruling, two cabinet members swiftly released statements vowing to protect the right to take medicines that had been approved by the federal government.“We stand unwavering in our commitment to ensure every American has access to health care and the ability to make decisions about health care — including the right to safe and legal abortion, such as medication abortion that has been approved by the F.D.A. for over 20 years,” Xavier Becerra, the secretary of Health and Human Services, said in his statement. In another statement, Merrick B. Garland, the attorney general, referred specifically to the first drug in the medication abortion regimen, mifepristone. In December, the F.D.A. made access to it significantly easier by permanently lifting the requirement that patients obtain mifepristone by visiting an authorized clinic or doctor in person.“We stand ready to work with other arms of the federal government that seek to use their lawful authorities to protect and preserve access to reproductive care,” Mr. Garland said. “In particular, the F.D.A. has approved the use of the medication mifepristone. States may not ban mifepristone based on disagreement with the F.D.A.’s expert judgment about its safety and efficacy.”But it is unclear what the Justice Department can do. Some legal scholars have argued that federal drug approval pre-empts state actions to restrict a drug’s use. Others say that has only applied to cases where a state claims that safety or efficacy is an issue.“Today, the Supreme Court said abortion, it can only be regulated in sort of a health and safety way when it’s permitted, but it can be completely banned,” Professor Watson said.As a result, she said, the ability of the federal government to assert that the F.D.A.’s approval takes precedence over state laws “is limited, given, traditionally, states get to regulate the practice of medicine.”Legal experts said there might be other ways for the Justice Department to become involved in fighting medication abortion restrictions, such as contesting laws that bar mailing pills, since the mail is under federal oversight.On Friday, the F.D.A. took a cautious stance, saying in a statement, “We have not had an opportunity to review the opinion, but we do note that F.D.A.’s independent and regulatory decisions are based on science and facts.”The agency added that “patients should have access to medications that are safe and effective for their F.D.A.-approved use.”Medication abortion became legal in the United States in 2000, when mifepristone was approved by the F.D.A. The agency imposed tight restrictions on the drug, many of which remain in place. But access to the method increased in 2016, when the F.D.A. expanded the time frame within which the drug could be taken — from seven weeks to 10 weeks into a pregnancy.Major medical groups cite years of data showing that medication abortion is safe. For example, a research program that the F.D.A. allowed to provide telemedicine consultations and send pills by mail reported that 95 percent of the 1,157 abortions that occurred through the program between May 2016 and September 2020 were completed without requiring any follow-up procedure. Patients made 70 visits to emergency rooms or urgent care centers, with 10 instances of serious complications, the study reported.As conservative states began passing more laws restricting access to surgical abortions, more patients opted for pills, especially because they could be taken in the privacy of one’s home.The Covid pandemic fueled that trend. The Guttmacher Institute, a research organization that supports abortion rights, reported that in 2020, medication abortion accounted for 54 percent of all abortions.As patients look for ways to obtain the pills, some are expected to turn to international websites like Aid Access, a European organization that the F.D.A. has tried — so far unsuccessfully — to stop from mailing pills to the United States, further complicating enforcement efforts.Mary Ziegler, a law professor at the University of California, Davis, who has written widely on abortion, said in an interview last month that there might be attempts by states that ban abortion to prosecute doctors and other health providers in other states who provide abortion services like consultations and pills to their residents, or to try to block organizations or funds that give financial help to patients to travel to other states.States where abortion remains legal are mobilizing to increase access stifle legal assaults from other states. Connecticut passed a bill that would prevent abortion providers from being extradited to other states, bar Connecticut authorities from cooperating with abortion investigations from a patient’s home state and allow Connecticut residents who are sued under another state’s abortion provision to countersue. Legislation in California would provide financial assistance to patients traveling from other states to obtain abortions and increase the number of abortion providers. Justice Brett Kavanaugh, in an opinion concurring with the Supreme Court decision, suggested that patients who traveled to other states to receive an abortion would be protected by the constitutional right to interstate travel.So far, most states that restrict abortion have long adhered to a principle of targeting providers and others who help patients, but not the patients themselves. Professor Ziegler said it was possible that could also change because, in circumstances where the abortion takes place outside state boundaries, “there may be absolutely no one else in that state to go after but the patient.”Katie Benner contributed reporting from Washington.
Read more →Trial of New Alzheimer’s Drug Reports Disappointing Results
The drug, crenezumab, failed to prevent early symptoms or slow cognitive decline, the latest setback in the long quest to find effective therapies for the disease.A closely watched clinical trial of a potential Alzheimer’s drug failed to prevent or slow cognitive decline, another disappointment in the long and challenging effort to find solutions for the disease.The decade-long trial was the first time people who were genetically destined to develop the disease — but who did not yet have any symptoms — were given a drug intended to stop or delay decline. The participants were members of an extended family of 6,000 people in Colombia, about 1,200 of whom have a genetic mutation that virtually guarantees they will develop Alzheimer’s in their mid-40s to mid-50s. For many members of the family, who live in Medellín and remote mountain villages, the disease has quickly stolen their ability to work, communicate and carry out basic functions. Many die in their 60s.In the trial, 169 people with the mutation received either a placebo or the drug, crenezumab, produced by Genentech, part of the Roche Group. Another 83 people without the mutation received the placebo as a way to protect the identities of people likely to develop the disease, which is highly stigmatized in their communities.The trial investigators had hoped that intervening with a drug years before memory and thinking problems were expected to emerge might hold the disease at bay and provide important insights for addressing the more common type of Alzheimer’s that is not driven by a single genetic mutation.“We’re disappointed that crenezumab did not show a significant clinical benefit,” Dr. Eric Reiman, the executive director of Banner Alzheimer’s Institute, a research and treatment center in Phoenix, and a leader of the research team, said at a news conference about the results. “Our hearts go out to the families in Colombia and to everyone else who would benefit from an effective Alzheimer’s prevention therapy as soon as possible. At the same time, we take heart in the knowledge that this study launched and continues to help shape a new era in Alzheimer’s prevention research.”The results are also another setback for drugs that target a key protein in Alzheimer’s: amyloid, which forms sticky plaques in the brains of patients with the disease. Years of studies with various drugs that attack amyloid in different stages of the disease have fallen flat. In 2019, Roche halted two other trials of crenezumab, a monoclonal antibody, in people in the early stages of the more typical Alzheimer’s disease, saying the studies were unlikely to show benefit.Last year, in a highly controversial decision, the Food and Drug Administration granted its first approval of an anti-amyloid drug, Aduhelm. The F.D.A. acknowledged that it was unclear if Aduhelm could help patients, but greenlighted it under a program that allows authorization of drugs with uncertain benefit if they are for serious diseases with few treatments and if the drugs affect a biological mechanism that is reasonably likely to help patients. The F.D.A. said that biological mechanism was Aduhelm’s ability to attack amyloid, but many Alzheimer’s experts criticized the decision because of the poor track record of anti-amyloid therapies. The trial results on Thursday only added to the disappointing evidence.Laura Cuartas caring for her son Dario, one of four of her adult children with Alzheimer’s disease.Todd Heisler/The New York Times“Wish there were something more positive to say,” said Dr. Sam Gandy, the director of Mount Sinai’s Center for Cognitive Health, who was not involved in the Colombia research.“The pathogenic mutation in the Colombian family is known to be involved in amyloid metabolism,” Dr. Gandy said, adding, “The thinking was that these were the patients most likely to respond to anti-amyloid antibodies.”Dr. Pierre Tariot, the director of the Banner Alzheimer’s Institute and a leader of the Colombian research, said some of the data did suggest that patients receiving crenezumab fared better than those receiving the placebo, but the differences were not statistically significant.He also said there were no safety problems with the drug, an important finding because many anti-amyloid therapies, including Aduhelm, have caused brain bleeding or swelling in some patients.Additional data from the trial will be presented at a conference in August. Dr. Tariot and Dr. Reiman noted that Thursday’s results did not include more detailed information from brain imaging or blood analysis of the drug’s effects on proteins and other aspects of the biology of Alzheimer’s. They also did not reflect increases in the dose of crenezumab, which researchers began giving to patients as they learned more about the drug, Dr. Tariot said. He said some patients received up to two years of the highest dose during the five to eight years they were in the clinical trial.Dr. Francisco Lopera, a Colombian neurologist and another leader of the research, began working with the family members decades ago and helped determine that their affliction was a genetic form of Alzheimer’s. He said the trial had convinced him that “prevention is the best way of looking for the solution for Alzheimer’s disease, even if today we don’t have a good result.”“We know that we did a big step in the contribution to the investigation of Alzheimer’s disease,” he added. “And now we are prepared to start other steps in looking at the solution for this disease.”One participant’s wife, Maria Areiza of Medellín, said her husband, Hernando, whose surname is being withheld to protect his privacy, was among the first patients to enroll in the trial. Hernando, 45, who worked fixing telephone cables, began developing symptoms of cognitive decline about eight years ago. He has since progressed to Alzheimer’s dementia but can still hold a conversation. Because his deterioration has been relatively slow, his family had been hopeful that he was benefiting from the trial.“I had put all my hopes in this study,” his wife said.Jennie Erin Smith contributed reporting from Medellín, Colombia.
Read more →New Experimental Therapy for A.L.S. Approved in Canada
The F.D.A. is also reviewing the treatment, Albrioza, but the agency’s scientists have raised questions about its effectiveness.An experimental therapy for A.L.S., the paralyzing and fatal neurological disorder, has been approved in Canada, adding a new treatment option for a disease for which there are few effective therapies.The approval, which comes with the condition that the drug company later provide better evidence that the treatment works, is likely to be of major interest to patients with A.L.S. (amyotrophic lateral sclerosis) in the United States, where the same therapy — AMX0035, to be marketed as Albrioza — is being evaluated by the Food and Drug Administration, which has raised questions about the treatment’s effectiveness.An F.D.A. review earlier this year found Albrioza to be safe, but said there was not enough evidence that it was effective either in helping patients live longer or slowing the rate at which they lose functions like muscle control, speaking or breathing without assistance. A committee of independent advisers to the F.D.A. voted by a narrow margin in March that the therapy was not ready for approval.The F.D.A. had been scheduled to issue a final decision this month, but recently extended the deadline to Sept. 29, saying it needed more time to review additional analyses of data submitted by the company.In the meantime, Calaneet Balas, president and chief executive of the A.L.S. Association, one of several patient advocacy organizations pressing for F.D.A. approval, said, “We expect that Americans living with A.L.S. will try to access Albrioza in Canada, just as we have heard reports of people trying to buy the ingredients on Amazon.” A.L.S., also called Lou Gehrig’s disease, is diagnosed in about 6,000 people worldwide each year and often causes death within two to five years. There are only two approved A.L.S. medications in the United States: riluzole, which can extend survival by several months, and edaravone, which can slow progression by about 33 percent.Albrioza is a combination of two existing drugs in the form of a bitter-tasting powder that is mixed with water and drunk or ingested through a feeding tube twice daily. It is produced by a small Massachusetts company, Amylyx Pharmaceuticals, whose founders, Justin Klee and Joshua Cohen, conceived of the therapy when they were undergraduate students at Brown University less than a decade ago.The Fight Against A.L.S.The illness, also called Lou Gehrig’s disease, robs people of their ability to move, speak, eat and ultimately breathe.Lacking Evidence: A new treatment held promise for slowing A.L.S., but an F.D.A. panel found that there’s not enough data to show that it works.A Runner’s Mission: After surpassing the average life expectancy for people with the disease, Andrea Peet decided to race a new kind of clock: 50 marathons in 50 states.Brain Implant: A man who is fully paralyzed by A.L.S. was able to communicate using only his thoughts.Rethinking Care: In 2017, Brian Wallach was diagnosed with A.L.S. Now, his startup aims to help other patients make the most of their time.Their idea was that combining taurursodiol, a supplement sometimes used to regulate liver enzymes, and sodium phenylbutyrate, a medication for a pediatric urea disorder, could safeguard neurons by preventing dysfunction of two structures in cells: mitochondria and the endoplasmic reticulum.The data comparing Albrioza to a placebo has so far come from one Phase 2 trial (which is smaller than the Phase 3 studies regulatory agencies generally require); additional information came from an open-label extension study that followed some patients after the trial ended, when they were knowingly taking the medication. The F.D.A. typically requires two persuasive clinical trials of a drug for approval, but in cases of severe disease with few available treatments, it can consider evidence from one clinical trial plus additional supporting data.A box of packets being used in a clinical trial of the two-drug combination AMX0035, which will be marketed as Albrioza.Aileen Perilla for The New York TimesHealth Canada greenlighted Albrioza under a program called Notice of Compliance with Conditions, which allows for approval of drugs that appear promising for serious diseases, but have incomplete evidence that they work. The central condition the agency set is that Amylyx “verify the clinical benefit of this drug” with data from a Phase 3 clinical trial that is underway and expected to conclude in 2024, according to agency documents sent to the company on Friday. The company must also conduct additional pharmacological studies and provide periodic safety reports. “Patients should be advised of the nature of the authorization,” the documents said.The F.D.A. has a similar program called accelerated approval that allows conditional approval of drugs with incomplete evidence of effectiveness, but that program also requires that a drug show that it targets part of the underlying biological mechanism of a disease. Experts have said that if Albrioza does not win standard F.D.A. approval, it would be unlikely to meet accelerated approval criteria because too little is known about the underlying biology of A.L.S., and how and whether Albrioza might address it.Last month, 38 doctors who treat A.L.S. patients sent a letter to the F.D.A. urging approval. The A.L.S. Association said its campaign for approval had in recent weeks generated more than 6,000 emails asking the agency to greenlight the drug.A member of the F.D.A.’s independent advisory committee, Dr. G. Caleb Alexander, who voted in March that there was insufficient evidence the therapy works, said he continues to think the F.D.A. should wait for the Phase 3 trial’s results and that “it would be a mistake to approve it based on the single trial alone.”Dr. Alexander, an internist and epidemiologist at the Johns Hopkins Bloomberg School of Public Health, said there was desperate need for effective therapies for A.L.S., but that for Albrioza, “it’s unfortunate, but the magnitude of unmet need is not matched by the quality of evidence to date.”He added that “approval in Canada could only further increase the pressure that the F.D.A. faces to rule favorably and to approve this product.”It is generally illegal for Americans to import drugs that haven’t been approved in the U.S. for personal use. But the F.D.A. website lists some exceptions that might apply to Albrioza, including if the drug has no serious safety issues and if it is to treat “a serious condition for which effective treatment is not available in the United States.”Dr. Angela Genge, director of the A.L.S. Global Centre for Excellence at the Montreal Neurological Institute, who has received fees from Amylyx for serving on an advisory board, said American patients would be legally able to receive Albrioza in Canada if it were prescribed by a Canadian physician and obtained from a Canadian pharmacy, though they would not be eligible for insurance coverage under Canada’s public or private system.In an interview, Mr. Cohen and Mr. Klee declined to disclose the price that Amylyx is considering for Albrioza, saying it was still being negotiated. They said that the therapy would be available in about six weeks for people who were paying privately, but would take longer, possibly months, for people to receive coverage under Canada’s public system. Amylyx has already been providing Albrioza at no cost under compassionate-use arrangements to 250 patients in the United States, they said.Until last summer, the F.D.A. had recommended that Amylyx not apply for approval until the drug had completed its Phase 3 trial, but in July, officials began suggesting that Amylyx submit an application for approval using existing data. The timing followed vociferous pressure from A.L.S. advocacy groups in the wake of the approval of the new Alzheimer’s drug, Aduhelm, which was controversial because many experts said there was insufficient data that Aduhelm worked.In the Phase 2 trial, two-thirds of the 137 participants received Albrioza, and over 24 weeks, they experienced a 25 percent slower decline than the participants receiving placebo — declining 2.32 points less on a 48-point A.L.S. scale that rates 12 physical abilities, including walking, speaking, swallowing, dressing, handwriting and breathing.The open-label extension study involved 90 of those patients, including 34 from the placebo group, who began taking the medication about seven months after those who had received it from the beginning. Those who received the treatment the longest had a median of 4.8 months more time before being hospitalized, being put on a ventilator or dying, Amylyx reported. Researchers involved in the study published more data last month that suggested additional benefit.Amylyx financed the bulk of its research on Albrioza, but the A.L.S. Association contributed $2.2 million, using money raised through the 2014 Ice Bucket Challenge. Amylyx has agreed to use sales of the drug to repay 150 percent of the association’s grant to fund more research.The clinical trials involved patients who developed symptoms within 18 months before the trial and were affected in at least three body regions, generally signs of fast-progressing disease. Health Canada’s approval did not place restrictions on which A.L.S. patients could take Albrioza, but the Amylyx founders and Dr. Genge said it is possible that such limitations might be established by the Canadian coverage system or by pharmaceutical formularies in some of Canada’s provinces.
Read more →1 in 5 Adult Covid Survivors in the U.S. May Develop Long Covid, Says CDC
One in five adult Covid survivors under the age of 65 in the United States has experienced at least one health condition that could be considered long Covid, according to a large new study by the Centers for Disease Control and Prevention. Among patients 65 and older, the number is even higher: one in four.In an indication of how seriously the federal health agency views the problem of long Covid, the authors of the study — members of the C.D.C.’s Covid-19 Emergency Response Team — recommended “routine assessment for post-Covid conditions among persons who survive Covid-19.”Long Covid is the term used to describe an array of symptoms that can last for months or longer after the initial coronavirus infection. The researchers identified post-Covid health problems in many different organ systems, including the heart, lungs and kidneys. Other issues involved blood circulation, the musculoskeletal system and the endocrine system; gastrointestinal conditions, neurological problems and psychiatric symptoms were also identified in the study.In both age groups, Covid patients had twice the risk of uninfected people of developing respiratory symptoms and lung problems, including pulmonary embolism, the study found. Post-Covid patients aged 65 and older were at greater risk than the younger group of developing kidney failure, neurological conditions and most mental health conditions.“It is sobering to see the results of this study again confirming the breadth of organ dysfunction and the scale of the problem,” said Dr. Ziyad Al-Aly, chief of research and development at the V.A. St. Louis Health Care System and a clinical epidemiologist at Washington University in St. Louis, who was not involved in the research.The study evaluated electronic medical records for nearly two million people — comparing those who had been infected with the coronavirus with those who were not. The most common post-Covid conditions, regardless of age, were respiratory problems and musculoskeletal pain.The risk of post-Covid patients aged 65 and older developing the 26 health conditions the study evaluated was between 20 percent and 120 percent greater than people who didn’t get Covid. Those aged 18 to 64 had a 10 percent to 110 percent greater risk than uninfected people of developing 22 of the health conditions. But in that age group, Covid survivors were no more likely than uninfected people to develop most mental health conditions, substance use disorders or strokes and similar cerebrovascular conditions.Dr. Al-Aly said the study results “can potentially translate into millions of people with new diabetes, heart disease, kidney disease, neurologic problems. These are lifelong conditions — certainly manageable, but not curable conditions.”The study analyzed records of 353,164 people who were diagnosed with Covid-19 in the first 18 months of the pandemic, beginning in March 2020. It compared them with the records of 1.64 million people who had a medical visit in the same month in which the Covid patients were diagnosed but did not become infected with the coronavirus during the study period, which ended on Oct. 31, 2021.People in both groups who had a history of one of the 26 health conditions in the previous year were excluded from the study — an attempt by the researchers to consider medical issues that patients developed only after they had Covid.The study, which involved patients seen at health facilities that use a record system managed by Cerner Corp., a large medical data company, said the Covid patients included people admitted to hospitals, seen in emergency departments or diagnosed in an outpatient setting. The researchers did not indicate how many patients were in each group, one of several limitations of the study’s findings.Between 30 days and 365 days after their coronavirus diagnosis, 38 percent of the patients experienced one or more new health problems, compared to 16 percent of the non-Covid patients, the study said. The younger age group, 18-to-64, was somewhat less likely to have those problems — 35 percent developed long Covid issues, compared with 15 percent of uninfected people. In the 65-and-older group, 45 percent had new health conditions, compared with 19 percent of uninfected people.Based on those percentages, the study authors calculated that nearly 21 percent of the younger group and nearly 27 percent of the older group developed health problems that could be attributed to long Covid.The study did not look at the vaccination status of the patients and did not report characteristics like race, ethnicity, sex or geographic location. It also did not identify which coronavirus variants were linked to each case.The C.D.C. authors concluded that post-Covid conditions might “affect a patient’s ability to contribute to the work force and might have economic consequences for survivors and their dependents.” They added that “care requirements might place a strain on health services” in “communities that experience heavy Covid-19 case surges.”Dr. Al-Aly said he agreed that people who had Covid should be medically evaluated for potential new health problems.“Now that we are in possession of knowledge that Covid-19 can lead to serious long-term consequences,” he added, “we need to develop additional tools to reduce the risk of long Covid.”
Read more →Do Vaccines Protect Against Long Covid?
Maybe, according to a growing number of studies, but there’s not yet a definitive answer.As the pandemic enters its third year, long Covid has emerged as an increasingly important concern. And many people are wondering whether getting a Covid shot can reduce their chances of developing long-term symptoms.What does the research show so far?The jury is still out, but a growing number of studies suggest that getting a Covid vaccine can reduce — though not eliminate — the risk of longer-term symptoms.The United Kingdom’s Health Security Agency conducted an analysis of eight studies that had been published on the topic before mid-January. It reported that six of the studies found that vaccinated people who became infected with the coronavirus were less likely than unvaccinated patients to develop symptoms of long Covid. The remaining two studies found that vaccination did not appear to conclusively reduce the chances of developing long Covid.How much protection could vaccines offer, according to the studies that found benefit?Some study results suggest substantial protection, while others find only a slight benefit.One large study of electronic records of patients in the U.S. Veterans Health Administration found that vaccinated Covid patients had only a 13 percent lower risk than unvaccinated patients of having symptoms six months later.Two studies in Britain found a bigger effect. One study of about 1.2 million people, based on patients’ reports via a phone app, found a 50 percent lower risk of lingering symptoms among vaccinated patients. Another, which has not been peer-reviewed and was based on surveying about 6,000 patients, found a 41 percent lower risk.A study of U.S. patients by Arcadia, a health care data firm, and the Covid Patient Recovery Alliance, a collaboration of leaders with health expertise in government and the private sector, found a larger benefit still. The study, which has not been peer-reviewed, analyzed records of about 240,000 patients infected with the coronavirus by May 2021 and found that those who had received even one dose of a Covid vaccine before their infection were one-seventh to one-tenth as likely to report two or more symptoms of long Covid 12 to 20 weeks later. That study also found that people who received their first vaccine dose after contracting the coronavirus were less likely to develop long Covid than those who remained unvaccinated, and the sooner they were vaccinated after infection, the lower the risk of long-term symptoms.A study in Israel, which has also not been peer-reviewed, found through surveys that people who received two doses of vaccine had between 54 percent and 82 percent lower risk than unvaccinated patients to report having seven of the 10 most common long-term symptoms. They were generally no more likely to report symptoms like headache, muscle pain and other issues than people in the general population who had never gotten Covid, the study said. (The authors said they could not confirm whether the patients were vaccinated before or after they had gotten Covid, but said that because of Israeli vaccination policy it was likely most people who received two doses of vaccine were infected with the coronavirus sometime after they had gotten their shots.)In the veterans’ study, also not yet published in a peer-reviewed publication, researchers compared about 48,000 patients who were unvaccinated when they got Covid with about 16,000 patients who were vaccinated. It found that vaccinated patients mostly benefited by being less likely to develop lung problems and blood-clotting difficulties, said one of the authors, Dr. Ziyad Al-Aly, chief of research and development at the V.A. St. Louis Health Care System and a clinical epidemiologist at Washington University in St. Louis. Other symptoms showed “very little risk reduction” from vaccines, he said.“The overall message is that vaccines reduce but do not eliminate the risk of long Covid,” said Dr. Al-Aly, adding that “reliance on vaccination as a sole mitigation strategy is wholly inadequate. It is like going to battle with a shield that only partially works.”A long-Covid patient was examined in a hospital in Tel Aviv in February.Amir Cohen/ReutersWhat about studies that don’t show any benefit?In an analysis of electronic medical records of patients in the United States, researchers in the United Kingdom compared about 10,000 people who had received Covid vaccines with a similar number of people who had not been vaccinated against the coronavirus but had received a flu vaccine — an effort to limit the number of people in the study who might be considered vaccine hesitant or who generally had less healthy behaviors.The study found that having a coronavirus vaccine before being infected did not reduce the risk of most symptoms of long Covid. There was some suggestion from the data that vaccinated people might be at lower risk of long-term symptoms like abnormal breathing and cognitive issues, the authors wrote, but those results were not statistically conclusive.The researchers said it was possible that because their data relied on electronic health records, the study might have captured only patients with the most severe symptoms, rather than a wider range of patients who did not seek medical attention for their symptoms.Why is the research conflicting?One reason is the differences in the studies themselves. Not all researchers have defined long Covid in the same way, measured the same symptoms or tracked patients for the same length of time. For example, some studies recorded symptoms that have lingered at least 28 days after infection, while others measured symptoms people were experiencing six months later. Studies relying on patient surveys may yield very different results than those based on electronic medical records. And some studies did not have very diverse populations. Patients in the veterans’ study, for example, were mostly older, white and male.Are the results different for different coronavirus variants?Much of the published data followed patients infected early in the pandemic. Some recently-published data included people infected by the highly contagious Delta variant, but it is too early for studies about vaccines and long Covid that include the Omicron variant. It’s also too early for studies evaluating the effect of boosters on long Covid.Is there anything scientists can conclude for sure?Yes. Vaccines are very effective at preventing people from getting seriously ill from infection by all the variants known so far. And many studies have found that Covid patients sick enough to be hospitalized were more likely to have lasting health issues. So, by keeping people out of the hospital, vaccines should reduce the chances of that type of long-term post-Covid case.Still, many people with long Covid had mild or even asymptomatic initial infections, and while some studies suggest vaccines have potential to ease their long-term symptoms, the evidence is not yet conclusive.Vaccines do offer some protection against getting infected to begin with — and avoiding infection, of course, is the surest way to prevent long Covid.Does the brand of vaccine make a difference in potential protection against long Covid?So far, studies have not found that different vaccines have different effects on long-term symptoms.What are the possible scientific reasons that vaccines might protect against long Covid?The cause of long Covid is still unclear, and different symptoms might have different underlying causes in different patients, scientists say. Some believe that the condition may be related to remnants of the virus or its genetic material lingering after the initial infection subsides. Another theory is that the continuing problems are related to inflammation or blood circulation problems spurred by an overactive immune response that is unable to shut down.Akiko Iwasaki, an immunologist at Yale, has said that vaccines may be able to provide lasting relief in people whose symptoms are caused by vestiges of the virus if the antibodies generated by the vaccines eliminate those remnants.But in people whose symptoms may be caused by a post-viral response resembling an autoimmune disease, she said, vaccines may help only temporarily, and problems like fatigue could re-emerge.One thing we know for certain is that vaccines are very effective at preventing people from getting seriously ill from infection by all the variants known so far. Kenny Holston for The New York TimesCan getting vaccinated help if you already have long Covid?When vaccines were first rolled out, some patients with long Covid were finding that symptoms like brain fog, joint pain, shortness of breath and fatigue improved after they had gotten vaccinated. Still, many people experienced no difference in their symptoms after vaccination, and a small percentage said they felt worse.A study by the Office for National Statistics in the United Kingdom found that in people ages 18 to 69 who reported their symptoms between February and September 2021, a first dose of a vaccine lowered the odds of reporting long Covid symptoms by 13 percent. A second dose further lowered the odds by 9 percent, the study found.The recent analysis by the U.K. Health Security Agency evaluated that study and seven others that examined whether vaccinating people with long Covid affected their symptoms. It found that in most of those studies, more people with long Covid reported improvement in their symptoms at some point after they were vaccinated. However, some people also reported worsening of symptoms, and in several studies the majority of people said their symptoms were unchanged.The agency noted that the definition of long Covid varied widely among the studies and that, because all the studies were observational, changes in symptoms could be due to factors other than vaccination.
Read more →