Orders for Morning-After Pills and Abortion Pills Rise After Trump’s Election
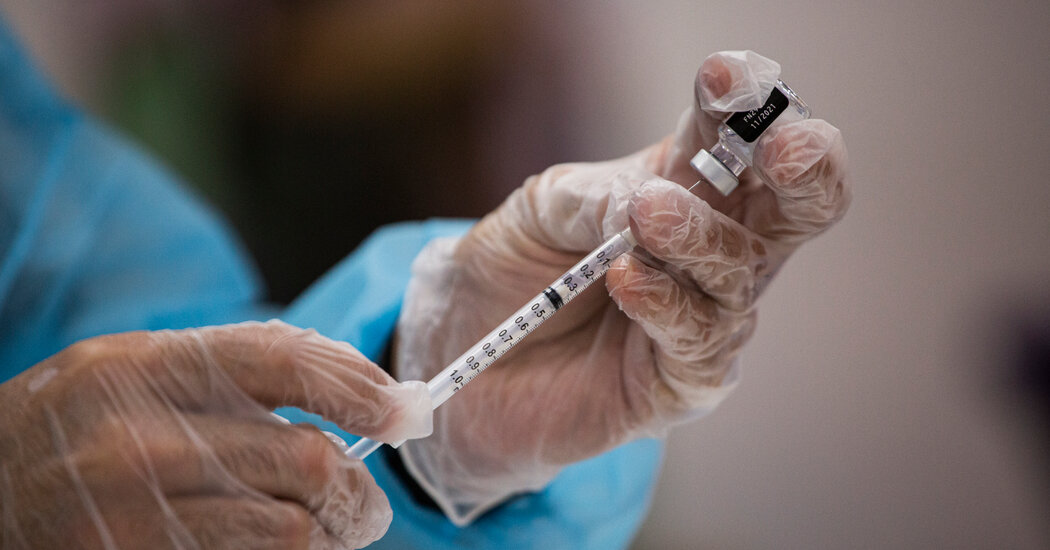
Some women are stocking up on the medications, saying they are concerned that the new administration could take steps to restrict access.The day after the election, Beth Ryan ordered two packages of emergency contraception pills and had them sent to her 27-year-old daughter.“We all felt so helpless,” said Ms. Ryan, who lives in Florida, describing her fear that a second Trump administration could further threaten access to reproductive health care. After one of her daughter’s friends, who handles online orders at Target, said he was seeing a burst of demand for Plan B morning-after pills, Ms. Ryan purchased Plan B from Walmart for her daughter in Colorado.“I think I felt better because I could control something,” she said. “I mean, it was something that you could do.”Across the country, some women are taking similar steps, ordering emergency contraceptives, abortion pills or both.There is no national data, but interviews with numerous providers of abortion and contraceptive drugs point to a surge in demand in the immediate aftermath of the election.Wisp, a telehealth provider of medications for reproductive health, said that during the five days after the election it sold more than 10,000 Plan B pills, which do not require a prescription; it sold fewer than 5,000 pills in the same Wednesday-through-Sunday span the week before the election.We are having trouble retrieving the article content.Please enable JavaScript in your browser settings.Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.Thank you for your patience while we verify access.Already a subscriber? Log in.Want all of The Times? Subscribe.
Read more →