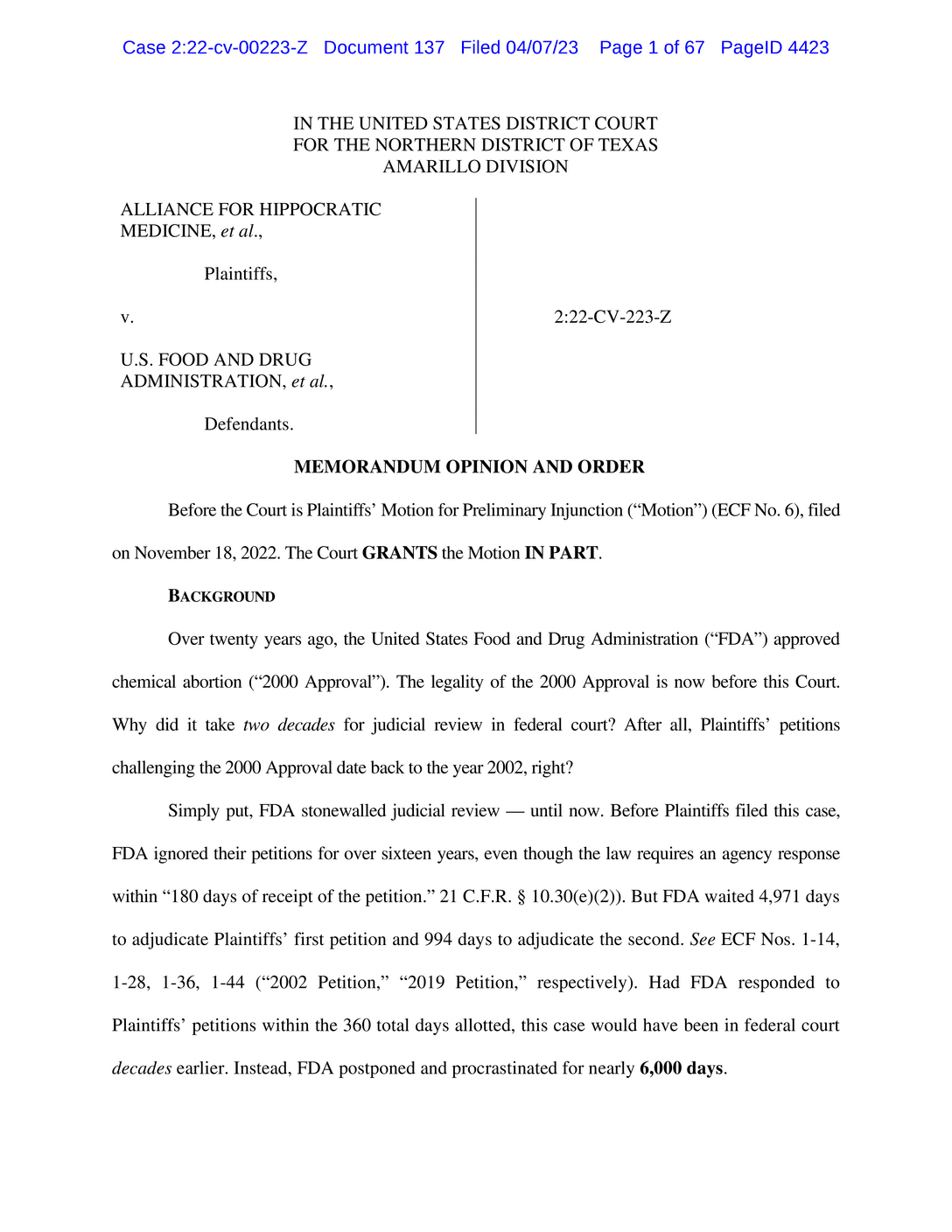
The Texas judge’s ruling was quickly contradicted by another federal judge in Washington State who ordered the F.D.A. to keep mifepristone available.A federal judge in Texas issued a preliminary ruling invalidating the Food and Drug Administration’s 23-year-old approval of the abortion pill mifepristone, an unprecedented order that — if it stands through court challenges — could make it harder for patients to get abortions in states where abortion is legal, not just in those trying to restrict it.The drug will continue to be available at least in the short-term since the judge, Matthew J. Kacsmaryk, stayed his own order for seven days to give the F.D.A. time to ask an appeals court to intervene.Less than an hour after Judge Kacsmaryk’s ruling, a judge in Washington state issued a ruling in another case, which contradicted the Texas decision, ordering the F.D.A. to make no changes to the availability of mifepristone in the 18 states that filed that lawsuit.The conflicting orders by two federal judges, both preliminary injunctions issued before the full cases have been heard, appear to create a legal standoff likely to escalate to the Supreme Court. President Biden said his administration would fight the Texas ruling. “This does not just affect women in Texas,” he said in a statement. “If it stands, it would prevent women in every state from accessing the medication, regardless of whether abortion is legal in a state.”The order by Judge Kacsmaryk, a Trump appointee who has written critically about Roe v. Wade, is an initial ruling in a case that could result in the most consequential abortion decision since the Supreme Court overturned Roe v. Wade last June.Read the Court Decision Invalidating F.D.A. Approval of MifepristoneA federal judge in Texas issued a preliminary ruling invalidating the Food and Drug Administration’s 23-year-old approval of the abortion pill mifepristone on Friday, clashing with another court’s ruling in a legal standoff likely to escalate to the Supreme Court.Read Document 67 pagesOn Friday night, the Justice Department filed a notice that it is appealing the Texas ruling.“Today’s decision overturns the F.D.A.’s expert judgment, rendered over two decades ago, that mifepristone is safe and effective,” Attorney General Merrick B. Garland said in a statement, adding that the Justice Department would ask that the decision be stayed while the appeal is pending. He said the department is reviewing the ruling in the Washington case.The lawsuit, filed by a coalition of anti-abortion groups and doctors, seeks to end more than 20 years of legal use of mifepristone, the first pill in the two-drug medication abortion regimen.The lawsuit in Washington State was filed against the F.D.A. by 18 Democratic attorneys general who were challenging the agency’s restrictions on the prescribing and dispensing of mifepristone. In a preliminary injunction in that case that he applied to the states that had sued, Judge Thomas O. Rice, who was appointed by President Barack Obama, blocked the agency from taking “any action to remove mifepristone from the market or otherwise cause the drug to become less available.”Read the Court Decision Ordering the F.D.A. to Keep Mifepristone AvailableA federal judge in Washington State issued a ruling ordering the Food and Drug Administration to make no changes to the availability of the abortion pill mifepristone on Friday, directly contradicting another judge’s ruling in a legal standoff likely to escalate to the Supreme Court.Read Document 31 pagesMedication abortion is the method used in more than half of abortions in the United States. The lawsuit claims that the F.D.A. did not adequately review the scientific evidence or follow proper protocols when it approved mifepristone in 2000 and that it has since ignored safety risks of the medication.The F.D.A. and the Justice Department have strongly disputed the claims in the lawsuit and said that the federal agency’s rigorous reviews of mifepristone over the years had repeatedly reaffirmed its decision to approve mifepristone, which blocks a hormone that allows a pregnancy to develop. In a statement Friday night, the agency said: “F.D.A. stands behind its determination that mifepristone is safe and effective under its approved conditions of use for medical termination of early pregnancy, and believes patients should have access to F.D.A.-approved medications that F.D.A. has determined to be safe and effective for their intended uses.”In the 67-page Texas ruling, Judge Kacsmaryk appeared to agree with virtually all of the claims made by the anti-abortion groups and repeatedly used the language of abortion opponents, calling medication abortion “chemical abortion” and referring to a fetus as an “unborn human” or “unborn child.””The court does not second-guess F.D.A.’s decision-making lightly,” the judge wrote. “But here, F.D.A. acquiesced on its legitimate safety concerns — in violation of its statutory duty — based on plainly unsound reasoning and studies that did not support its conclusions. There is also evidence indicating F.D.A. faced significant political pressure to forgo its proposed safety precautions to better advance the political objective of increased ‘access’ to chemical abortion.”Are Abortion Pills Safe? Here’s the Evidence.The Times reviewed 101 studies of medication abortion, spanning continents and decades. All concluded that the pills are a safe method for terminating a pregnancy.A lawyer for Danco Laboratories, which makes the branded version of mifepristone, called Mifeprex, and had joined the lawsuit on the side of the F.D.A., forcefully disagreed with the judge’s characterizations.“The court’s ruling rewrites the facts and the law to tell its preferred narrative — which is a story line that conflicts with established legal principles and with Mifeprex’s well-established safety profile,” the lawyer, Jessica Ellsworth, said in a statement. Danco filed a notice that it was appealing the ruling.Erik Baptist, a lawyer for the anti-abortion groups that filed the Texas case called the decision “a significant victory for the doctors and medical associations we represent, and more importantly, the health and safety of women and girls.” Mr. Baptist, who is senior counsel for the Alliance Defending Freedom, a conservative Christian legal organization, said, “by illegally approving dangerous chemical abortion drugs, the F.D.A. put women and girls in harm’s way, and it’s high time the agency is held accountable for its reckless actions.”Legal experts said that even if the Texas ruling is ultimately upheld, there would be several legal options that could allow the manufacturers of mifepristone to continue supplying the drug and providers to continue prescribing it to patients.A still taken from a livestream of Judge Matthew Kacsmaryk’s nomination hearing to the federal bench in 2017.U.S. Senate Committee on the JudiciaryShortly after the rulings on Friday night, the chief executive of GenBioPro, one of the two manufacturers of mifepristone in the United States, issued a statement saying the company was reviewing the decisions of both judges.“We will take any steps necessary to lawfully make mifepristone available and accessible to as many people as possible in the country,” the statement from the C.E.O., Evan Masingill, said.And if legal access to mifepristone is blocked, some abortion providers plan to provide only the second abortion medication, misoprostol, which is used safely on its own in many countries where mifepristone is less available. Misoprostol, a drug that is approved for other medical uses, causes contractions similar to a miscarriage and is considered slightly less effective on its own than in combination with mifepristone and more prone to cause side effects like nausea.Where Restrictions on Abortion Pills Could Matter Most in the U.S.A lawsuit is challenging the F.D.A.’s approval of medication abortion. Data shows the consequences could be far-reaching.In the Texas lawsuit, the plaintiffs also seek to ban the use of misoprostol for abortion, but their request for a preliminary injunction focused on mifepristone.Since last year’s Supreme Court ruling overturning the national right to abortion, the pills used in medication abortions have increasingly become the focus of political and legal battles. Some conservative states, in addition to banning or restricting abortion in general, have begun considering legislation that specifically targets abortion pills. And several recent lawsuits have been filed in efforts to preserve or expand access to medication abortion.The lawsuit filed in Washington state was intended to be a direct challenge to the Texas case. The Democratic attorneys general filed the case in late February on the first day that Judge Kacsmaryk could have issued a ruling. While its main claims sought to eliminate a framework of extra restrictions that the F.D.A. has long applied to mifepristone, the suit also asked the judge to declare that the F.D.A.’s “approval of mifepristone is lawful and valid” and to enjoin the F.D.A. “from taking any action to remove mifepristone from the market or reduce its availability.”In a news conference earlier this week, Washington’s attorney general, Bob Ferguson, characterized the lawsuit he and the other attorneys general filed as “the opposite of what’s going on in Texas.” He added “So the potential is there for two decisions or judges that are, in effect, contrary to one another. In other words, one judge in Texas could potentially say ‘Hey I’m issuing a ban on mifepristone nationwide’ and a judge in Washington State in the case with 17 other states could say ‘no, no, not only is it available, you got to expand access to it.’”People marched in Amarillo, Texas, in February to protest the lawsuit seeking to force the withdrawal of the F.D.A.’s approval of mifepristone.Meridith Kohut for The New York TimesThe case has caused a frenzy of concern in the reproductive health community. It was filed by the Alliance for Hippocratic Medicine, an organization that lists five anti-abortion groups as its members and was incorporated in August in Amarillo, Texas, where the case was filed. Judge Kacsmaryk is the only federal judge covering the Amarillo division in the U.S. District Court of the Northern District of Texas. The F.D.A. has regulated mifepristone more stringently than many other drugs and has regularly reviewed evidence for its safety and effectiveness.For a dozen years, the agency has imposed an additional framework of restrictions and monitoring for the drug. Called a Risk Evaluation and Mitigation Strategy, or REMS, that framework has been used for only about 300 other drugs, only 60 of which it currently covers.In recent years, the F.D.A. has extensively reviewed new data on mifepristone and has lifted several of the restrictions, including the requirement that patients obtain the drug in person from a provider.Some of the same anti-abortion organizations that filed the Texas lawsuit had previously filed, in 2002 and 2019, citizen petitions opposing the F.D.A.’s actions on mifepristone. Both were rejected by the agency as unfounded. And a 2008 review by the Government Accountability Office found no irregularities in the F.D.A.’s mifepristone approval.Legal experts said that the ruling appeared to be the first time that a court had acted to order that a drug be removed from the market over the objection of the F.D.A. and that if the ruling stood, it could have repercussions for federal authority to regulate other types of drugs.In his statement, Mr. Biden said, “If this ruling were to stand, then there will be virtually no prescription, approved by the F.D.A., that would be safe from these kinds of political, ideological attacks.”Adam Liptak
Read more →