MRI scan could screen men for prostate cancer
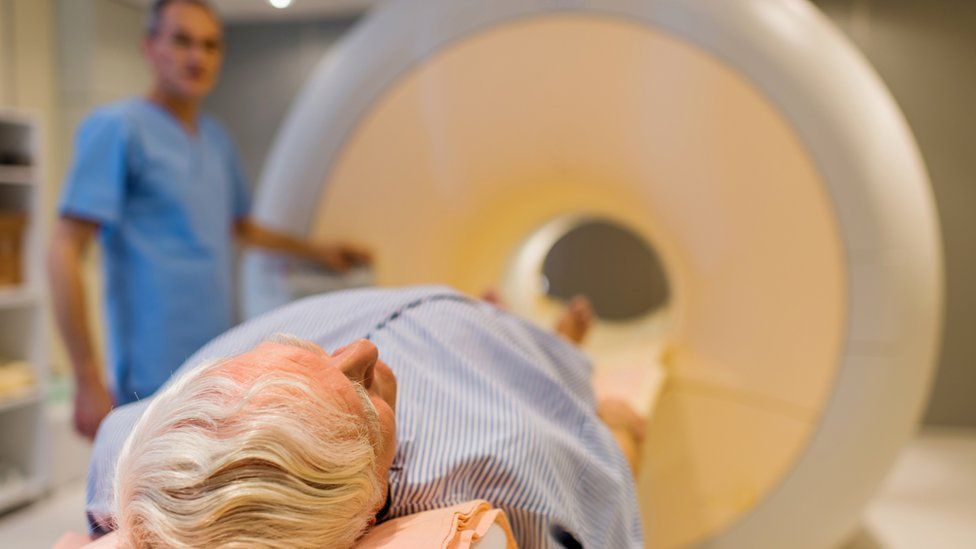
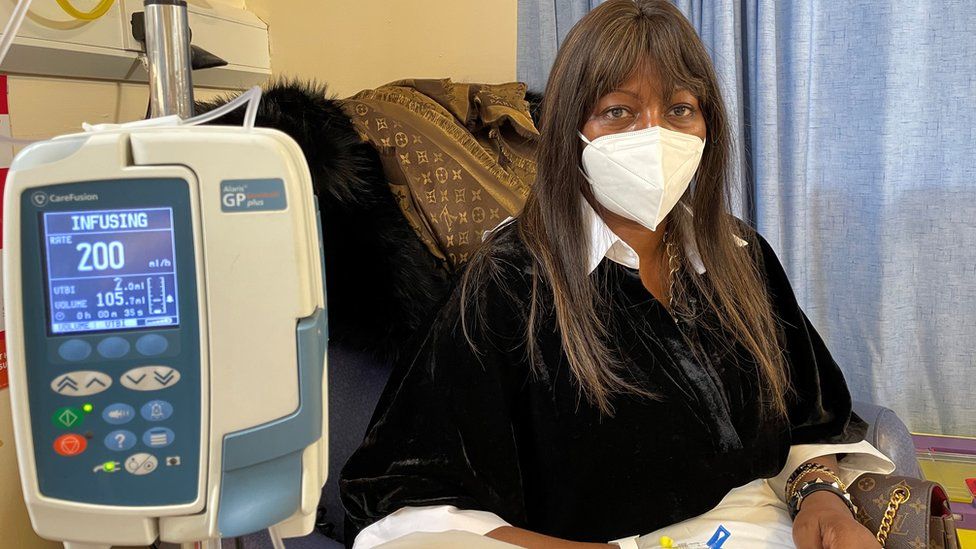
Published23 minutes agoShareclose panelShare pageCopy linkAbout sharingImage source, Getty ImagesBy Fergus WalshMedical editor A 10-minute MRI scan could be used to screen men for prostate cancer, according to a new study.The scans proved far more accurate at diagnosing cancer than blood tests, which look for high levels of a protein called PSA.MRI picked up some serious cancers that would have been missed by PSA alone.At present there is no national screening programme because PSA is considered too unreliable, although men over 50 can request a PSA test. What is prostate cancer?Part of the male reproductive system, the prostate gland, about the size of a walnut, is in the pelvis, below the bladderIt surrounds the urethra – the tube that takes urine out of the body through the penisCancer is abnormal and uncontrolled cell growthBut in the prostate, it usually develops slowlyThere may be no signs or symptoms for yearsAnd some never develop any problems from itBut in others, the cancer can be aggressive and deadlyEarly diagnosis and treatment is keyFor the Reimagine study, which is published in BMJ Oncology, men aged 50 to 75 in London were invited for screening MRI and PSA tests, which were carried out at University College Hospital.Of the 303 who had both tests, 48 had a positive MRI that indicated cancer and of these 25 were diagnosed with significant cancer after further tests, including biopsies. More than half the men whose cancer was picked up on MRI had low PSA test scores below 3ng/ml, which is considered normal, and so would have been falsely reassured they were free of disease.Prof Caroline Moore, consultant urologist UCLH and chief investigator of the study at University College London, said: “Our results give an early indication that MRI could offer a more reliable method of detecting potentially serious cancers early, with the added benefit that less than 1% of participants were ‘over-diagnosed’ with low-risk disease.” Image source, UCLHPaul Rothwell, 62, had his prostate cancer diagnosed as a result of being on the trial. It was caught early and he was successfully treated. He feels fortunate because his PSA test was negative and so would have given false reassurance had it not been for his MRI. Paul, from Hertfordshire, told the BBC: “If I’d just had the blood test I would be carrying on life as normal walking around unaware that there was some sort of ticking time bomb inside me of a cancer slowly growing, and by the time I did find out, presumably it would have been much harder to treat and much more dangerous to me.”PSA tests are considered useful but unreliable indicators of prostate cancer. High levels, which can indicate cancer, can also be caused by a recent infection or vigorous exercise and sex. This can lead to overdiagnosis of cancer and, as the trial showed, a low PSA score might miss cancer. The authors of the study suggest that prostate MRI could be used for screening, though they say a larger study would be needed to assess this. For the trial, black men were five times less likely to come forward for screening than white men, even though they have a higher risk of prostate cancer. Saran Green, another study author from King’s College London, said: “One in four black men will get prostate cancer during their lifetime, which is double the number of men from other ethnicities. Given this elevated risk, it will be crucial that any national screening programme includes strategies to reach black men and encourage more of them to come forward for testing.”Errol McKellar, 66, from Essex, was diagnosed with prostate cancer 13 years ago. After successful treatment he returned to work as a car mechanic and began offering customers a discount if they got their, or their partner’s, prostate checked.He now runs a charity, the Errol McKellar Foundation, which aims to raise awareness of prostate cancer and to ensure more men come forward for testing.He told the BBC: “When they brought their car in I’d ask men, ‘When was the last time you had a service and MOT on yourself?’.”There’s a massive mistrust of the medical system among the African-Caribbean community, and that has to be dealt with. But also there’s two elements that we find that come up very often: one is fear, and the other one is ignorance.”When prostate cancer turns up at your front door, it doesn’t care whether you’re black, whether you’re white – if you ignore it, it will kill you. In the end this is about all men, and leaving no-one behind.”Prof Mark Emberton, senior author of the study, said a screening programme could be up and running within the next decade: “The UK prostate cancer mortality rate is twice as high as in countries like the US or Spain because our levels of testing are much lower. Given how treatable prostate cancer is when caught early, I’m confident that a national screening programme will reduce the UK’s prostate cancer mortality rate significantly.”Simon Grieveson, assistant director of research at Prostate Cancer UK, said: “When a man’s prostate cancer is caught early, it’s very treatable. Sadly, more than 10,000 men each year are diagnosed too late, when their cancer has already spread. “MRI scans have revolutionised the way we diagnose prostate cancer, and it’s great to see research into how we might use these scans even more effectively. These results are extremely exciting, and we now want to see much larger, UK-wide studies to understand if using MRI as the first step in getting tested could form the basis of a national screening programme.”What symptoms should people check for?The common ones are:needing to urinate more frequently – particularly at nightdifficulty starting to urinate, weak flow and it taking a long timeblood in urine or semenThese symptoms can be caused by other conditions too – but it is important to have any changes checked by a doctor.More on this storyMen left embarrassed by urinary incontinence tabooPublished1 day agoBBC presenter Nick Owen reveals cancer diagnosisPublished7 AugustYearly prostate tests advisable for some menPublished20 October 2021Prostate screening could be ready in five yearsPublished27 December 2021Turnbull saved lives, says prostate cancer charityPublished2 September 2022Cancer patient urges men to get prostate testedPublished14 JuneRelated Internet LinksBMJ OncologyUniversity College London Hospitals NHS Foundation TrustThe BBC is not responsible for the content of external sites.
Read more →