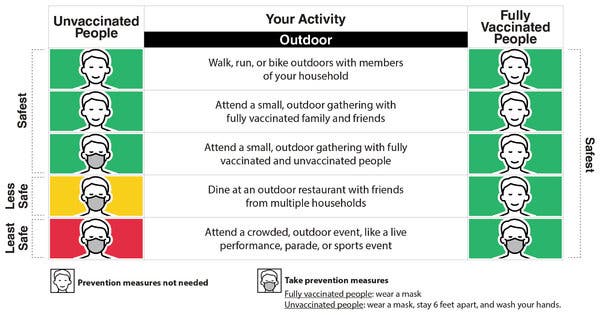
CDC Details New Mask Advice for Vaccinated People
Americans who are fully vaccinated against the coronavirus no longer need to wear masks outdoors if they’re walking, running, hiking or biking alone, with members of their household, or if they attend small outdoor gatherings, federal health officials announced on Tuesday.The Centers for Disease Control and Prevention stopped short of telling those people that they could shed their masks altogether in outdoor settings — citing the worrying risk that remains for transmitting the coronavirus, unknown vaccination levels among people in crowds and the still high-caseloads in some regions of the country.Federal health officials and President Biden were announcing the updated advice on Tuesday, linking the news with the administration’s public campaign to get most American adults vaccinated by summer and trying to offer reassurances that some semblance of normal life can return.But the C.D.C. is maintaining advice on other safety measures, saying vaccinated adults should continue wearing masks and staying six feet apart in large public spaces, like outdoor performance or sports events, indoor shopping malls and movie theaters, where the vaccination and health status of others would be unknown. And they still should avoid medium and large gatherings, crowds and poorly ventilated spaces, officials said.“I welcome less restrictive guidelines about masking outdoors,” said Linsey Marr, an aerosol scientist at Virginia Tech. “We know that transmission outdoors is much less likely to occur than indoors, because the virus cannot accumulate in the air outdoors. It’ll become rapidly diluted.”But the guidelines themselves, which outline different masking recommendations for a variety of scenarios, seem overly complex, she said. “I can’t remember this. I would have to carry around a sheet of paper — a cheat sheet with all these different stipulations.” She added: “I worry that this is not as helpful as it could be.”Americans have been whipsawed on the issue of mask-wearing advice since the beginning of the pandemic, when top health officials said people did not need them — in part because of severe shortages of protective gear for health care workers on the front lines.And mask restrictions since then have been a patchwork from state to state, despite growing evidence of a mask’s protection for individuals and those around them. Many states have already lifted restrictions they had put in place for indoor and outdoor activities. Others like New York, however, have maintained mask-wearing requirements even for outdoor spaces, citing the threat of potentially more contagious variants.But the pace of vaccinations has helped influence some easing of those limits. So far, about 42 percent of Americans have received at least one dose of a Covid-19 vaccine, and 29 percent have received both doses of the two vaccines requiring double shots.Centers for Disease Control and PreventionThe vaccines are highly effective at preventing people from becoming seriously ill from the coronavirus.“Scientifically the vaccines are good enough that it’s highly unlikely that someone who’s vaccinated is going to be exposed to enough virus outdoors to have a breakthrough infection,” Dr. Marr said.Early evidence also suggests that vaccinated people may be significantly less likely to transmit the virus, but the exact risks are not yet known.Some experts also wondered if the new directives were confusing, by establishing different standards for those who are vaccinated and those who are not, even though it is impossible to know who is who.“It’s not like you can go up to someone in public and say, ‘You don’t have a mask on – are you vaccinated?’” said Dr. Monica Gandhi, an infectious disease specialist at the University of California, San Francisco. “Those who aren’t vaccinated will promptly take their mask off outdoors because no one can check.”But, she said, that is probably fine, since the risk of transmission in outdoor settings is very low, absent close or prolonged contact with someone.Dr. Mercedes Carnethon, an epidemiologist at Northwestern University, said the relaxed guidelines signal that “if you’re outside in a group of individuals who you know well, then it is safe to be without a mask if you were vaccinated. I don’t think that it goes so far as to change what our behavior needs to be in outdoor settings where we don’t know people, and we can’t distance.”Masking and distancing are still generally recommended when gathering with unvaccinated people from more than one other household or with an unvaccinated person who is at high risk of severe illness from Covid, or who lives with a vulnerable person.And there are scenarios in which wearing a mask outdoors can still be an important social signal, Dr. Carnethon said. For instance, no vaccine has yet been authorized for children under 16. “And when we’re going to require children to wear masks, at school and on the playground when they’re at school,” she said, “I think that it is responsible for the adults in the situation to model that behavior and normalize mask wearing even when outside.”A growing body of research indicates that the risk of spreading the virus is far lower outdoors than indoors. Viral particles disperse quickly outdoors, experts say, meaning brief encounters with a passing walker or jogger pose very little risk of transmission.But most if not all of the research about viral transmission outside was done before the vaccine was available.A recent systematic review of studies that examined the transmission of the coronavirus and other respiratory viruses among unvaccinated individuals concluded that fewer than 10 percent of infections occurred outdoors and that the odds for indoor transmission were 18.7 times higher than outdoors. (The odds of super-spreading events were 33 times higher indoors.)
Read more →