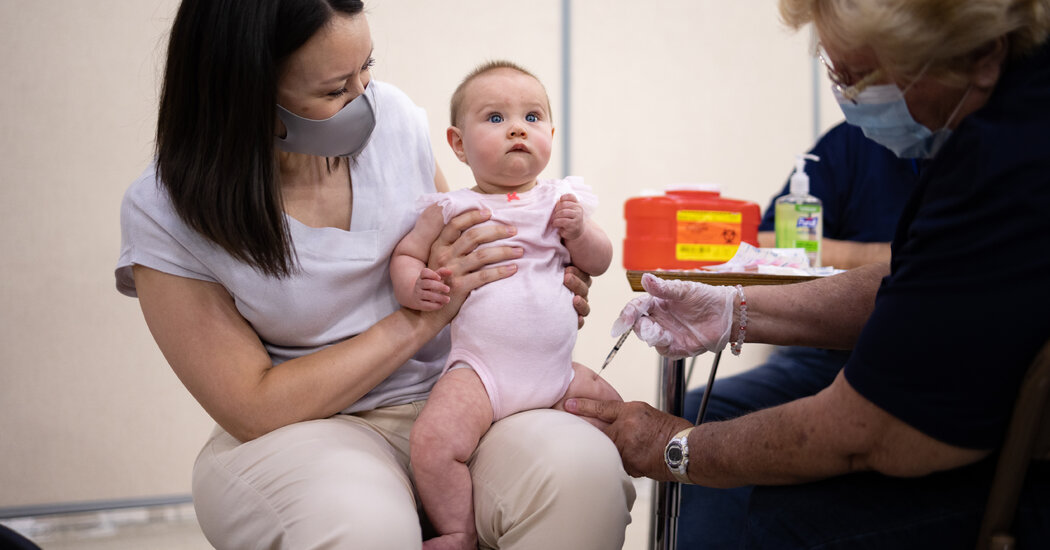
The soaring drug fatalities in the U.S. are being fueled partly by fentanyl-tainted pills bought by teenagers and young adults on Snapchat, Instagram, TikTok and other social media apps.Shortly after Kade Webb, 20, collapsed and died in a bathroom at a Safeway Market in Roseville, Calif., in December, the police opened his phone and went straight to his social media apps. There, they found exactly what they feared.Mr. Webb, a laid-back snowboarder and skateboarder who, with the imminent birth of his first child, had become despondent over his pandemic-dimmed finances, bought Percocet, a prescription opioid, through a dealer on Snapchat. It turned out to be spiked with a lethal amount of fentanyl.Mr. Webb’s death was one of nearly 108,000 drug fatalities in the United States last year, a record, according to preliminary numbers released this month by the Centers for Disease Control and Prevention. Law enforcement authorities say an alarming portion of them unfolded the same way as his: from counterfeit pills tainted with fentanyl that teenagers and young adults bought over social media.“Social media is almost exclusively the way they get the pills,” said Morgan Gire, district attorney for Placer County, Calif., where 40 people died from fentanyl poisoning last year. He has filed murder charges against a 20-year-old man accused of being Mr. Webb’s dealer, who pleaded not guilty. “About 90 percent of the pills that you’re buying from a dealer on social media now are fentanyl,” Mr. Gire said.The phenomenon has led to disturbing new statistics:Overdoses are now the leading cause of preventable death among people ages 18 to 45, ahead of suicide, traffic accidents and gun violence, according to federal data.Although experimental drug use by teenagers in the United States has been dropping since 2010, their deaths from fentanyl have skyrocketed, to 884 in 2021, from 253 in 2019, according to a recent study in the journal JAMA.A Drug Enforcement Administration fact sheet shows authentic prescription pills and counterfeit ones.Drug Enforcement AdministrationRates of illicit prescription pill use are now highest among people ages 18 to 25, according to federal data.Much as drug dealers in the 1980s and ’90s seized on pagers and burner phones to conduct business covertly, today’s suppliers have embraced modern iterations — social media and messaging apps with privacy features such as encrypted or disappearing messages. Dealers and young buyers usually spot each other on social media and then often proceed by directly messaging each other.The platforms have made for a swift, easy conduit during the coronavirus pandemic, when demand for illicit prescription drugs has jumped, both from anxious, bored customers and from those already struggling with addiction who were cut off from in-person group support.The Opioid CrisisFrom powerful pharmaceuticals to illegally made synthetics, opioids are fueling a deadly drug crisis in America.Origins of an Epidemic: Purdue Pharma knew OxyContin was widely misused, but continued to promote the painkiller as less addictive.An Unrelenting Surge: Deaths from drug overdoses again rose to record-breaking levels in 2021, nearing 108,000, according to the Centers for Disease Control and Prevention.A Settlement: Purdue Pharma reached a deal with a group of states that long resisted the structure of the original bankruptcy plan. Here is what the agreement means.Detailing Tragedies: As part of the settlement, families who lost loved ones to opioid addiction were allowed to address the owners of Purdue Pharma in court.How Opioids Work: Through interviews with users and experts, we created a visual representation of how these drugs hijack the brain.Supplies of tainted pills, crudely pressed by Mexican cartels with chemicals from China and India, have escalated commensurately. Fentanyl, faster and cheaper to produce than heroin and 50 times as potent, made for a highly addictive filler. Last year, the federal Drug Enforcement Administration seized 20.4 million counterfeit pills, which experts estimate represent a small fraction of those produced. Its scientists say that about four out of 10 pills contain lethal doses of fentanyl.The result is that new waves of customers are swiftly becoming addicted, said Dr. Nora Volkow, director of the National Institute on Drug Abuse. “When you are putting fentanyl in pills that are sold as benzodiazepines or for pain, you are reaching a new group of customers that you wouldn’t have if you were just selling fentanyl powder.”A photo of Mr. Webb from a vacation he took in Kauai, Hawaii.Brandon Thibodeaux for The New York TimesZachariah Plunk, 17, of Mesa, Ariz., died after buying a counterfeit Percocet from a dealer he reached through Snapchat.via Wendy PlunkIn a two-month span in the fall, the D.E.A. identified 76 cases that involved drug traffickers who advertised with emojis and code words on e-commerce platforms and social media apps. The agency has included a feature in its One Pill Can Kill public awareness campaign: a poster called “Emoji Drug Code: Decoded,” with images of drug symbols.“There are drug sellers on every major social media platform — that includes Instagram, Facebook, Twitter, Snapchat, Pinterest, TikTok and emerging platforms like Discord and Telegram,” said Tim Mackey, a professor at the University of California San Diego who runs a federally funded start-up that developed artificial intelligence software to detect illicit online drug sales. “It’s an entire ecosystem problem: As long as your child is on one of those platforms, they’re going to have the potential to be exposed to drug sellers.”At around 1:30 a.m. on Aug. 15, 2020, Zachariah Plunk, 17, a star high school football player from Mesa, Ariz., contacted a dealer through Snapchat, seeking a Percocet.As footage from the family’s home security camera would reveal, the dealer dropped off drugs around 3 a.m. Zach went outside, swallowed a pill and fell to the curb. At 5 a.m., a 15-year-old neighbor found him dead.To Wendy Plunk, Zach’s mother, the ease with which dealers can evade detection is particularly devastating. The man who sold her son the fatal pill remains on Snapchat, she said, adding, “I keep an eye on the guy. Every time he gets kicked off, he changes his name a bit and gets on again, with the same picture.”In January, parents of children as young as 13 who had died from pills protested in front of Snapchat’s headquarters in Santa Monica, Calif., with signs accusing the company of being an accomplice to murder. One speaker was Laura Berman, a relationship therapist and television host. In February 2021, her 16-year-old son, Sam, bought what he thought was a Xanax through a Snapchat connection, ingested it and died at home of fentanyl poisoning.Facing a barrage of criticism from law enforcement and grieving parents, social media platforms have been stepping up policing on their sites, shutting down dealers’ accounts and redirecting drug seekers to addiction services.In January, family and friends of young people who died after taking counterfeit pills protested outside Snapchat’s headquarters in Santa Monica, Calif.Apu Gomes/Agence France-Presse — Getty ImagesOn Monday, the Ad Council announced a wide-ranging campaign to roll out this summer, funded by three tech companies — Snap, Meta and Google — to alert teenagers and young adults about the dangers of fentanyl. Social media platforms like Twitter, TikTok, Twitch and Reddit are expected to provide landing zones for the warnings.Snap, the parent company of Snapchat, and Meta, the parent of Instagram and Facebook, report they are increasingly interrupting drug exchanges. Snap said it took action on 144,000 drug-related accounts in the United States from July to December last year. That figure doesn’t include the 88 percent of drug-related content that was pre-emptively detected by artificial intelligence software, which monitors terms that could signify drug deals.Now, when Snapchat users search for “fenta,” “xanax” or other drug language, the results are blocked. They are redirected to an in-app video channel with content from nonprofit groups and the C.D.C. that addresses “fentapills” — the dangers of purported OxyContin, Percocet, Xanax and Adderall.According to Facebook’s latest community standards report, it took action on four million drug-related exchanges worldwide in the fourth quarter of 2021. Instagram took action on 1.2 million, figures which represent alerts from both users and pre-emptive detection technology.On Instagram, one recent search for Percocet did set off an automatic warning and an offer of help. But it also yielded numerous results, including an account that posted photos of the pills and contact information, with phone numbers on the encrypted messaging apps Wickr and WhatsApp.And when companies remove dealers from their platforms, many sellers simply leapfrog to another.“We detect about 10,000 new drug-related accounts a month,” said Dr. Mackey, whose software company detects illicit online drug trafficking for private and public organizations.Most drug seekers will not baldly search for a drug by name, he said. They may use a hashtag with a celebrity associated with it. Enterprising dealers troll comments for customers, inserting themselves in online exchanges among seekers of pain relief.During the pandemic, drug use has surged as mental health among young adults and teenagers has deteriorated, studies show. Young people tend to eschew heroin, not only because of its addictive properties but also because of a skittishness about syringes, say experts in adolescent behavior. Pills, with the false imprimatur of medical authority, appear safer. Moreover, to their generation, prescription medications — for anxiety, depression and focus — have become normalized.Ed and Mary Ternan of Pasadena, Calif., in their son Charlie’s bedroom.Michael Tyrone Delaney for The New York TimesCharlie Ternan died after taking what he thought was a Percocet he bought from a dealer through Snapchat.Michael Tyrone Delaney for The New York Times“By the time the kid goes to college, his friends all have prescription bottles in their backpacks — they’re used to sharing pills,” Ed Ternan said. “The drug traffickers know that.” In May, 2020, his 22-year-old son, Charlie, three weeks away from college graduation, bought what he thought was a Percocet for back pain from a dealer he connected with on Snapchat. Thirty minutes after ingestion, Charlie, 6-foot-2 and 235 pounds, was dead from fentanyl poisoning.Rather than sue Snap for wrongful death, Mr. Ternan and his wife, Mary, asked the company to step up monitoring.“I said: ‘If the kids were buying real Percocet on Snapchat, they wouldn’t be dying. You guys need to escalate this problem right up to the same level as child sex trafficking,’” Mr. Ternan said.The Ternans formed Song for Charlie, one of many organizations of families who have lost children to fentanyl. Mr. Ternan has met with federal officials and has connected Snapchat with digital and drug treatment experts. His group creates cautionary content for TikTok and Snapchat.The rules of engagement in the war on drugs have shifted, Mr. Ternan said, adding: “It’s now about chemistry and social media distribution and encryption. We need different kind of generals, a more collaborative approach between Big Tech and the government.”To fine-tune prevention messaging, Snap commissioned Morning Consult, the digital market research firm, to conduct a survey of drug knowledge. The results, from a random sample of 1,449 Snapchat users ages 13 to 24, underscore their vulnerability to misusing prescription drugs. They expressed feeling overwhelmed, anxious and depressed but also fearful of the stigma surrounding mental health challenges. “Coping with stress” was the top reason to turn to illicit pills, they said.But only half the respondents overall, and 27 percent of the teenagers, knew that fentanyl could be in counterfeit pills. When asked to rate the danger posed by certain drugs, nearly two-thirds were likely to rank heroin and then cocaine as “extremely dangerous,” but scarcely a third put fentanyl in that category. Overall, 23 percent didn’t even know enough about fentanyl to rank its danger level, including 35 percent of adolescents.That ignorance is what drove Wendy Thomas, a substitute third-grade teacher from Sanford, N.C., to repurpose her grief over the 2020 death of her son from a counterfeit Percocet, and use it to reach teenagers. With her nonprofit, Matthew’s Voice, she has written health-class curriculums about fentanyl for high school freshmen and seventh-graders that are currently under final review by a large North Carolina school district.Signs showing photos of people who died after being poisoned by pills containing fentanyl, before a protest outside Snap in June 2021.Patrick T. Fallon/Agence France-Presse — Getty ImagesIt also motivates anguished parents like Elizabeth Dillender, who is Kade Webb’s mother and the grandmother of his newborn daughter, Indigo Kade. “I’m not naïve enough to think that social media is going away,” she said. “We have to work in conjunction with social media to get the word out to these kids.”Ms. Dillender has taken her campaign to Spotify, where she has a fentanyl awareness podcast, and to social media platforms like TikTok and Facebook.Recently, her podcast featured Laura Didier, another mother from Mr. Webb’s hometown, Rocklin, Calif. A year before Mr. Webb died, Ms. Didier’s former husband found their 17-year-old son, Zach, in his bedroom slumped lifeless over his computer keyboard. Zach had bought what he thought was a Percocet from a dealer on Snapchat.“You think if there’s a problem, you’ll see red flags — their grades are dropping, their disposition and friends are changing,” Ms. Didier reflected recently. “But that’s old thinking about drug behavior. This can happen so quickly without your ability to predict. I just don’t want families to be complacent and think, It can’t happen to us.”To underscore that message, at least one harm reduction network, the Santa Clara Opioid Overdose Prevention Project in California, has been promulgating the darkly instructive warning: #ExpectFentanyl.
Read more →