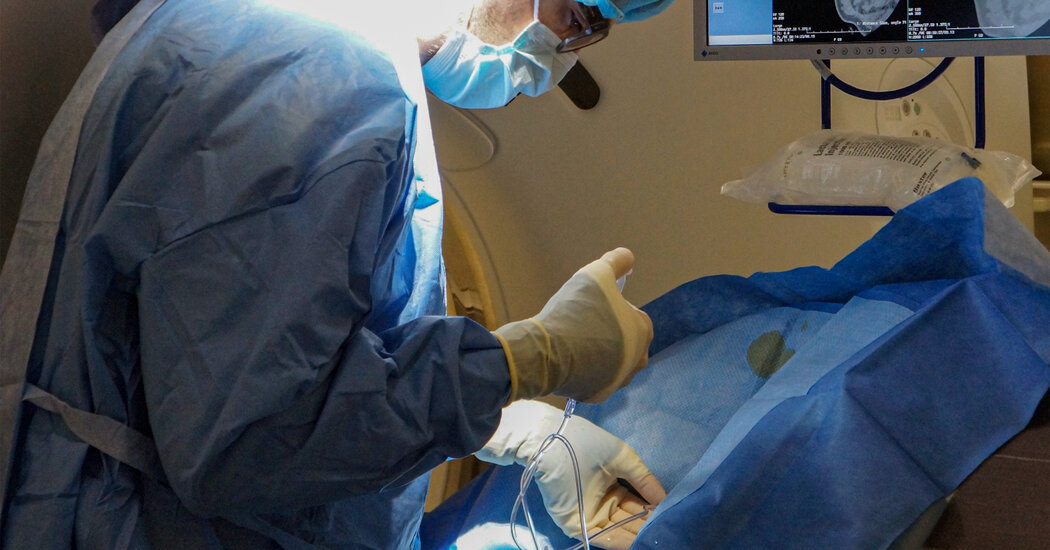
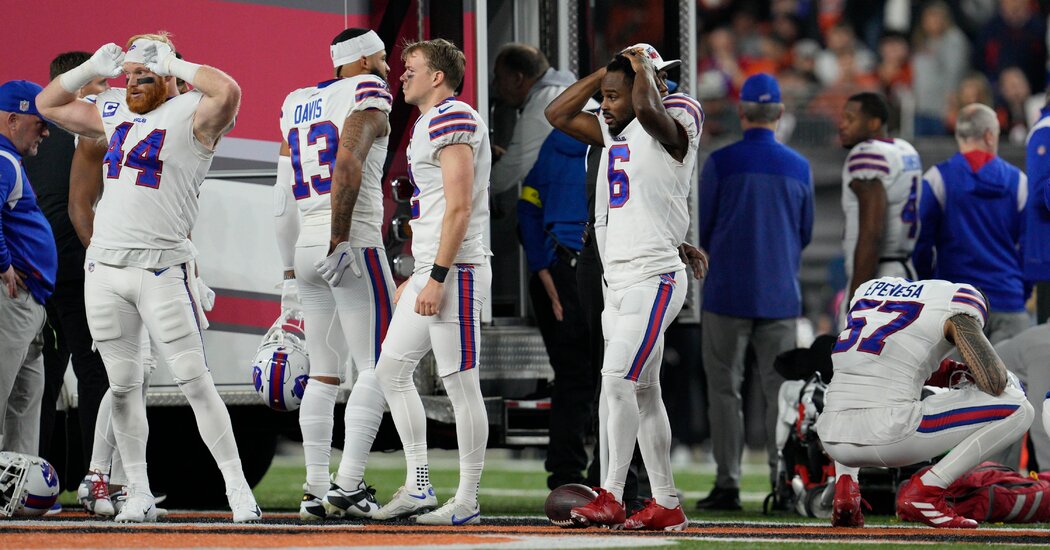The trauma care of the Buffalo Bills player highlighted what is done to overcome cardiac arrest, a leading cause of death in the United States.CINCINNATI — Damar Hamlin, the Buffalo Bills player whose heart stopped during a game in Cincinnati on Jan. 2, should not have survived, if statistics on cardiac arrests are any guide.Mr. Hamlin “was dead,” when he fell to the ground, said Dr. Timothy A. Pritts, chief of the section of general surgery at the University of Cincinnati Medical Center, where Mr. Hamlin was treated.But the 24-year-old safety left the hospital a week after his cardiac arrest with no apparent neurological deficits. He beat the odds after a stunning incident that traumatized his loved ones, teammates, opponents and tens of millions of Monday Night Football viewers. A visit to the hospital and the doctors, nurses and other medical staff who helped bring him back to life highlighted the mix of good preparation and good fortune that allowed Mr. Hamlin to escape a leading cause of death in the United States.Cardiac arrest, when the heart stops, is distinct from a heart attack, which occurs when blood flow in an artery feeding the heart is blocked. Outside of a hospital, more than 300,000 people a year have a cardiac arrest, also known as sudden cardiac death. The survival rate for those who have cardiac arrests outside of hospitals and, like Mr. Hamlin, have bystander cardiopulmonary resuscitation, is just 11.2 percent. For the few like Mr. Hamlin who receive immediate defibrillation, survival rises to 41 percent.Mr. Hamlin’s doctors said they were unable to discuss many of the particulars of his case, but they were able to describe the procedures they use to treat patients like him.Minutes count.“A few extra minutes or even a few extra seconds and it could have been a different outcome,” said Dr. William Knight IV, an emergency medicine and trauma specialist at the medical center.Brain damage is likely if the person in cardiac arrest goes 4 to 6 minutes without CPR, and brain death occurs after 10 minutes. Only 8 percent of cardiac arrest survivors emerge with a good neurological outcome. Most, according to Monica Sales, a spokeswoman for the American Heart Association, “have some degree of brain injury.”Damar Hamlin of the Buffalo Bills “was dead” when he fell to the ground after a hit during a game on Jan 2.Greg M. Cooper/Associated PressMr. Hamlin’s treatment began on the field at Paycor Stadium.Joshua A. Bickel/Associated PressImmediate CPR and defibrillation by medical personnel at the football game who responded rapidly is “absolutely certainly” what saved Mr. Hamlin’s life and his brain, said Dr. Benjamin Levine, professor of medicine and cardiology at the University of Texas Southwestern Medical Center and Texas Health Dallas.Dr. Levine and Dr. Jeremy Cannon, a trauma and critical care specialist at the University of Pennsylvania, emphasized the paramount importance of rapid response to cardiac arrest. Medical research to improve outcomes for cardiac arrest patients now focuses on ways to teach the public that CPR and use of a defibrillator are easy and can be learned in minutes and that many 911 operators can walk them through the procedures. The idea is to empower people to save lives.Damar Hamlin’s CollapseThe Buffalo Bills safety went into cardiac arrest during an N.F.L. game in Cincinnati on Jan. 2. He was released from the hospital on Jan. 11.Injury and Reaction: The life-threatening injury to Damar Hamlin, which was televised on “Monday Night Football,” resonated around the league and the world of sports.His Recovery: Hamlin, who returned home nine days after his collapse, appears to be on a good path for neurological recovery, though doctors said it was too early to know how far he is from returning to normal life.In His Hometown: As the news about Hamlin became more hopeful, anguish turned to “happy tears” in the tight-knit Pennsylvania borough where he grew up.Emergency Response: When Hamlin’s heart stopped, medical personnel could be heard making clear the severity of his condition and the efforts to keep him alive. Listen to the audio.CPR has changed as well. Now, it is “hands only”— no more mouth-to-mouth.“Mission critical No. 1 is blood flow to the brain,” said Dr. Charles J. Prestigiacomo, a neurosurgeon at the University of Cincinnati. The brain is the neediest organ, requiring 15 to 20 percent of the body’s blood.People are now taught to press hard on the chest 100 times a minute — singing “Staying Alive,” by The Bee Gees will give the right rhythm.But research on how to improve the odds for cardiac arrest patients has languished, according to Dr. Benjamin Abella, a resuscitation expert and emergency physician at the University of Pennsylvania. Impediments include little national data reporting, a paucity of funding and a lack of accountability for hospitals’ outcomes for said patients.Mr. Hamlin’s treatment began on the field at Paycor Stadium, where the game was being played.The National Football League and its teams contract with Level 1 trauma centers — medical centers that can provide the most comprehensive care — near every stadium where they play. The University of Cincinnati Medical Center sends seven physicians to every Bengals home game. The center also sends paramedics, respiratory therapists and an ambulance crew.The University of Cincinnati’s trauma center sends several medical personnel to every Bengals home game: concussion watchers, emergency doctors, paramedics, respiratory therapists and an ambulance crew.Maddie McGarvey for The New York TimesA trauma room at the University of Cincinnati Medical Center emergency departmentMaddie McGarvey for The New York TimesAs soon as Mr. Hamlin fell to the ground on Jan. 2, that medical team rushed to the field, communicating by radio because the stadium was so loud it was impossible to hear one another speak. The air “was vibrating” with sound, said Dr. Brett Kissela, a neurologist at the medical center who was at the game.And thus it began — an elaborate process of treating a trauma patient that requires “teams of teams,” Dr. Pritts said. In the first few hours, a severe trauma patient like Mr. Hamlin is physically touched by as many as 50 people. By the end of the first 24 hours, that number swells to 100 people.The medical center, founded in 1823, works with the U.S. Air Force to train military trauma physicians and medical teams. Its emergency department treats around 4,800 trauma patients a year.Those who were at the ready when Mr. Hamlin came in are doctors, nurses and other medical professionals who have seen the worst of the worst. Every patient who arrives in the surgical trauma intensive care unit — where Mr. Hamlin was treated — “is having the worst day of their life,” Dr. Pritts said.The staff members are deeply affected by their work with trauma patients.“When I go home, I need down time. I sit by myself for 15 minutes to decompress,” said Michele Hodge, a nurse who manages the medical center’s emergency department.Hospital employees are quick to credit Mr. Hamlin’s recovery to his youth and health. But they also attribute their intricately choreographed care and experience to having an average of five cardiac arrest patients each week.Ashleigh Schmeltzer, a CT scanner technologist, said she is reminded of the crews at the Indianapolis 500 that swarm to a car needing attention.In the emergency room, “everyone has a job and a role,” she said.“When I go home I need down time. I sit by myself for 15 minutes to decompress,” said Michele Hodge, a nurse who manages the emergency department.Maddie McGarvey for The New York TimesAshleigh Schmeltzer, a C.T. scanner technologist, said she is reminded of Indianapolis 500 crews that swarm to a car needing attention. “Everyone has a job and a role,” she said.Maddie McGarvey for The New York TimesThe first team that responds to a case like Mr. Hamlin’s includes a “doc head,” who is an airway specialist and stands at the patient’s head, and a “doc foot,” the team leader, who stands at the patient’s feet. A respiratory therapist stands at one side of the patient’s head, and a supervising airway doctor stands at the other. Two nurses and two other doctors stand on either side of the patient, while a scribe stands to the side and writes everything down. Two additional doctors stand to the side of the stretcher.Within minutes, the team wheels the patient to an adjacent room for a rapid whole body CT scan by staff members like Ms. Schmeltzer.A CT scan is so fast — taking minutes — and so accurate “it’s like eyes looking into the body,” said Dr. Mary Mahoney, professor of radiology at the medical centerA scan can’t give doctors every bit of information they want but, Dr. Mahoney said, it is invaluable to the trauma team. “It can point you in the right direction.” It can show areas that where fluid is accumulating and can show, for example, if blood is pooling in the sac around the heart.Although Mr. Hamlin’s heart was beating again by the time he reached the emergency room, he had a common complication of a cardiac arrest known as acute respiratory distress syndrome, or A.R.D.S.Because of A.R.D.S., Mr. Hamlin needed to spend most of his time lying face down. When a patient has A.R.D.S., it typically means fluid has seeped out of the blood vessels and accumulated in lung tissue. Doctors have learned that patients with A.R.D.S. are more likely to get the oxygen they need and survive if they lay face down for about 16 hours each day and on their backs for the other 8 hours. The prone position, said Dr. Amy Makley, the medical director of trauma, shifts the fluid in the lungs.“We prone patients as long as they need it,” Dr. Makley said, which meant, in Mr. Hamlin’s case, from the time he arrived in the intensive care unit until the time his doctors were able to wean him from a ventilator five days later.During that time, cooling pads were placed on Mr. Hamlin’s chest and thighs to chill his body. The doctors’ hope was that lowering body temperature to about 92.3 degrees would help to protect the brain because chemical reactions that can damage injured cells slow down as body temperature falls. But patients’ bodies try to shiver, which raises the temperature, so they must be sedated or given paralytic agents.Mr. Hamlin was already sedated to allow him to tolerate a ventilator. He was kept chilled until his ventilator was removed.A hallway of the emergency department.Maddie McGarvey for The New York TimesFor the first few days in the unit, Mr. Hamlin’s doctors worried about whether he would recover at all and, if so, to what extent.But on Jan. 4, they said in a news conference at the hospital, Mr. Hamlin had begun to improve. He was awake enough to communicate by nodding and shaking his head. To the medical staff’s delight, he even wrote, “did we win?” on a pad provided by a bedside nurse.Finally, a week after his cardiac arrest, hospital staff secreted him out of the medical center to fly back to Buffalo. Dr. Knight accompanied him to the Cincinnati airport.It still is not known why Mr. Hamlin had a cardiac arrest. A likely explanation was a rare event, commotio cordis, in which a blow to the chest — in his case, from a tackle — at exactly the right 20-millisecond interval in the heart’s cycle can make the heart stop. But Mr. Hamlin’s doctors still need to eliminate other possible causes for his injury, like a heart defect. Sometimes, they never find a cause.The staff at the medical center insists that all patients are treated the same — from the 30 percent who are uninsured to the wealthy donors to celebrities.Of course, though, Mr. Hamlin was different.“We’ve taken care of his illness before, but what do you do when you have to drive past 20 interview trucks?” asked Dr. Stewart Wright, the hospital’s chief medical officer.Flowers and cards for Mr. Hamlin arrived by the truckload, and donated meals were constantly being delivered. Fans attached posters to a chain-link fence outside, flew balloons and held candlelight vigils.There were so many callers that the medical center had to hire additional operators, but the hospital would not even confirm to callers that he was a patient.Now, the crowds and the attention are gone. The hospital is back to normal, and its staff is breathing sighs of relief for Mr. Hamlin. Back in Buffalo, he faces what could be weeks to months of recuperation.“This is the beginning of the next stage of his recovery,” Dr. Knight said.He added that he was starting his own recovery from Mr. Hamlin’s episode.“I’m exhausted,” he said.“That was the longest week in my professional career.”
Read more →