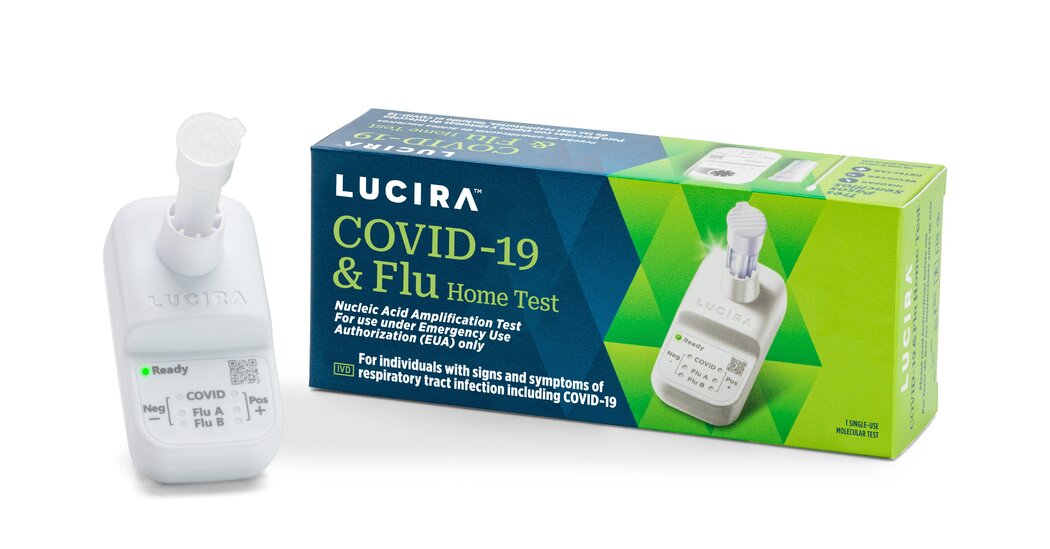
First At-Home Test for Flu and Covid Is OK’d by the FDA
But the company that created the 30-minute, over-the-counter test has filed for bankruptcy, so the product’s eventual availability to consumers remains unclear.The Food and Drug Administration issued an emergency authorization for the first over-the-counter, at-home combination flu and Covid test on Friday, just two days after the company that makes the test announced that it had filed for bankruptcy protection based, in part, on the agency’s lengthy approval timeline.The single-use test works with a self-collected nasal swab and provides a result in about 30 minutes, according to the F.D.A. The test is meant to be used by people 14 and older, or by an adult collecting a sample from someone age 2 or older.The test’s developer, Lucira Health, based in California’s Bay Area, announced its bankruptcy plan on Wednesday, noting that it had expected its emergency-use authorization for the test in August before the onslaught of the flu season. The company said the agency’s authorization process “became protracted,” and said it had high expenditures as it moved forward with manufacturing the combination tests.Without revenue that the company expected from projected sales of the tests during this year’s flu season, Lucira decided that it would pursue a sale of its business but continue operations to serve customers, according to its news release. The bankruptcy plan was reported earlier in The Wall Street Journal.In a statement issued on Friday, Dr. Jeff Shuren, the director of the F.D.A.’s device division, called the test “a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home.”But even though public health experts and scientists welcomed the test authorization, it remained unclear when such a combined test would be widely available for sale to consumers. And that uncertainty compounded concerns that others have voiced about the Biden administration’s plans to end the coronavirus public health emergency in May, which could complicate access to testing.People with private insurance and those on Medicare, who have been eligible for eight free at-home tests per month, may have to pay out of pocket for the tests once the emergency ends.Erik Engelson, Lucira’s chief executive, said in a statement Friday that the company was “very excited” about the authorization. “I can’t thank our employees and partners enough for seeing this through, and of course, for the F.D.A.’s recognition,” he said.Lucira Health did not immediately respond to questions about its manufacturing capacity or how much the test would cost consumers. The combination test correctly identified 99 percent of negative and 90 percent of positive flu A samples, according to the F.D.A. It also detected 100 percent of the negative and 88 percent of the positive Covid samples. The agency said it expected the company to continue to test on the flu B strain, which was not prevalent this year.The product is a molecular test, which means it detects and amplifies the genetic material of the viruses, as a P.C.R. test does. These tests are generally more sensitive than antigen tests, and at-home molecular tests have been more expensive than rapid antigen tests. The new test will be the first of a series of combination diagnostics in different stages of development that will scan for multiple ailments at once, said Dr. Wilbur Lam, a pediatric hematologist and bioengineer at Emory University who has helped federal officials with test development and validation.“Now we’re in this new era that’s honestly pretty exciting,” Dr. Lam said. “It’s exciting for a health care provider, it’s exciting for the technology developers, and I think exciting for the public because we have this new test. And this is only the beginning.”Through the pandemic, some public health experts have criticized the F.D.A. for being slow to approve at-home Covid tests and the federal government for failing to make the tests more widely available to Americans at little or no cost. Even once at-home tests were approved, fluctuating demand prompted manufacturers to ramp down production, contributing to shortages of rapid tests when the virus came surging back.During the first years of the pandemic, flu activity was unusually low. But last fall, with most pandemic precautions gone, the flu re-emerged in alarming numbers so early in the flu season. Over the last several months, Americans have had to contend with waves of multiple viruses, including influenza, the coronavirus and respiratory syncytial virus, or R.S.V.
Read more →