NY AG Urges Stricter Asthma Drug Warnings Due to Children’s Mental Health Risks
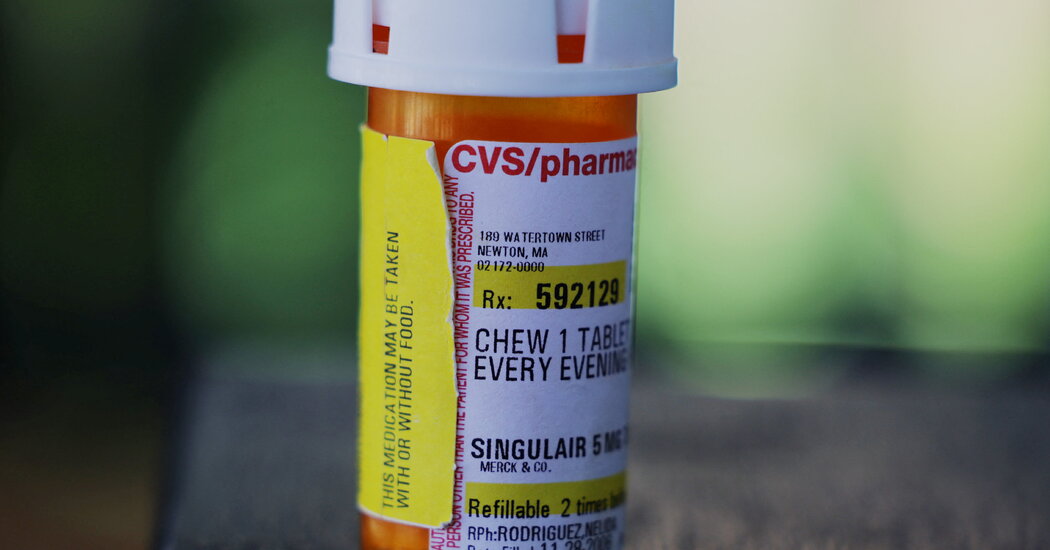
The A.G., Letitia James, called on the F.D.A. to redouble efforts to alert doctors about potential side effects of Singulair and to consider discouraging use of the drug in children.The New York attorney general on Thursday urged the Food and Drug Administration to “take immediate action” and renew alerts to doctors and patients about the dangerous effects of Singulair for children, saying that the current warnings about the drug’s psychiatric side effects were not sufficient.In a letter, the attorney general, Letitia James, also called on the federal agency to consider discouraging the prescription of Singulair, an asthma and allergy drug, to children.Thousands of patients and parents have complained to the F.D.A. about symptoms of anxiety, rage, hallucinations and other psychiatric problems that they linked to the drug, which is also known in its generic form as montelukast. Those reports, combined with an emotional F.D.A. hearing in 2019 and cases cited in medical literature, led the F.D.A. in 2020 to order its most stringent warning on instructions for the drug’s usage.But an examination by The New York Times found that people continued to report that they were not aware of the possible side effects, which include suicide or suicide attempts, when they took the medication or gave it to their children.Ms. James cited The Times’s article, and called on the F.D.A. “to implement new, more stringent safety regulations for the drug,” particularly for children.“Parents and guardians have the right to be fully informed of a medication’s potential side effects when making choices about their children’s health,” Ms. James said in a statement on Thursday. “The risks associated with taking Singulair are far too dire to come without a very clear warning.”We are having trouble retrieving the article content.Please enable JavaScript in your browser settings.Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.Thank you for your patience while we verify access.Already a subscriber? Log in.Want all of The Times? Subscribe.
Read more →