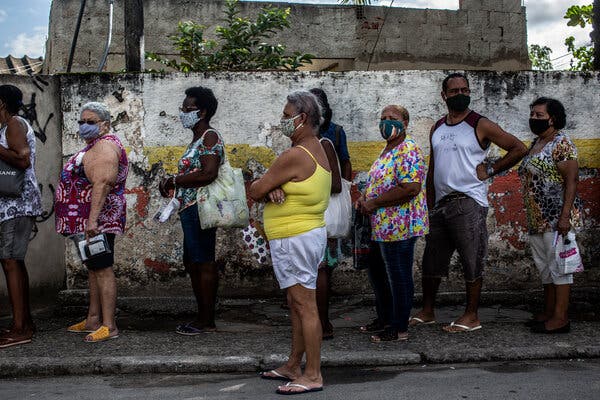Declining infection rates over all masked a rise in more contagious forms of the coronavirus. Vaccines will stop the spread, if Americans postpone celebration just a bit longer.For weeks, the mood in much of the United States has been buoyant. Cases, hospitalizations and deaths from the coronavirus have fallen steeply from their highs, and millions of people are being newly vaccinated every day. Restaurants, shops and schools have reopened. Some states, like Texas and Florida, have abandoned precautions altogether.In measurable ways, Americans are winning the war against the coronavirus. Powerful vaccines and an accelerating rollout all but guarantee an eventual return to normalcy — to backyard barbecues, summer camps and sleepovers.But it is increasingly clear that the next few months will be painful. So-called variants are spreading, carrying mutations that make the coronavirus both more contagious and in some cases more deadly.Even as vaccines were authorized late last year, illuminating a path to the pandemic’s end, variants were trouncing Britain, South Africa and Brazil. New variants have continued to pop up — in California one week, in New York and Oregon the next. As they take root, these new versions of the coronavirus threaten to postpone an end to the pandemic. At the moment, most vaccines appear to be effective against the variants. But public health officials are deeply worried that future iterations of the virus may be more resistant to the immune response, requiring Americans to queue up for regular rounds of booster shots or even new vaccines.“We don’t have evolution on our side,” said Devi Sridhar, a professor of public health at the University of Edinburgh in Scotland. “This pathogen seems to always be changing in a way that makes it harder for us to suppress.”Seniors wait in line to receive vaccinations in Belford Roxo, Brazil. A variant first found in the country has appeared in North America.Dado Galdieri for The New York TimesHealth officials acknowledge an urgent need to track these new viruses as they crawl across the United States. Already, B.1.1.7, the highly contagious variant that walloped Britain and is wreaking havoc in continental Europe, is rising exponentially in the United States.Limited genetic testing has turned up more than 12,500 cases, many in Florida and Michigan. As of March 13, the variant accounted for about 27 percent of new cases nationwide, up from just 1 percent in early February.The Biden administration has pledged a “down payment” of $200 million to ramp up surveillance, an infusion intended to make it possible to analyze 25,000 patient samples each week for virus variants. It’s an ambitious goal: The country was sequencing just a few hundred samples each week in December, then scaling up to about 9,000 per week as of March 27.Until recently, B.1.1.7’s rise was camouflaged by falling rates of infection over all, lulling Americans into a false sense of security and leading to prematurely relaxed restrictions, researchers say.“The best way to think about B.1.1.7 and other variants is to treat them as separate epidemics,” said Sebastian Funk, a professor of infectious disease dynamics at the London School of Hygiene and Tropical Medicine. “We’re really kind of obscuring the view by adding them all up to give an overall number of cases.”Other variants identified in South Africa and Brazil, as well as some virus versions first seen in the United States, have been slower to spread. But they, too, are worrisome, because they contain a mutation that diminishes the vaccines’ effectiveness. Just this week, an outbreak of P.1, the variant that crushed Brazil, forced a shutdown of the Whistler Blackcomb ski resort in British Columbia.A patient brought into the Royal London Hospital in Britain in January. A surge of infections mostly caused by a more contagious variant was difficult to bring under control. Andy Rain/EPA, via ShutterstockThe world is caught in a sprint between vaccines and variants, and the shots eventually will win, scientists say. But because each infection gives the coronavirus a chance to evolve still further, vaccinations in the United States and elsewhere must proceed as fast as possible.Infections are rising again, driven to an uncertain degree by B.1.1.7 and other variants. Earlier this week, Dr. Rochelle Walensky, director of the Centers for Disease Control and Prevention, pleaded with Americans to continue to practice masking and social distancing, saying she felt a sense of “impending doom.”“We have so much to look forward to — so much promise and potential of where we are and so much reason for hope,” she said. “But right now I’m scared.”‘More infectious for more days’The coronavirus was supposed to be slow to change shape. Like all viruses, it would pick up mutations and evolve into thousands of variants, scientists said at the beginning of the pandemic. But it would not change significantly for years — a stupid virus, some called it.The pathogen defied those predictions. “We expected the virus to change,” said Dr. Michael Diamond, a viral immunologist at Washington University in St. Louis. “We didn’t quite anticipate how quickly it was going to occur.”A variant is of concern only if it is more contagious, causes more severe disease, or blunts the immune response. The variants identified in Britain, South Africa, Brazil and California all fit the criteria.B.1.1.7, the first to come to widespread attention, is about 60 percent more contagious and 67 percent more deadly than the original form of the virus, according to the most recent estimates.The variant is no different from the original in how it spreads, but infected people seem to carry more of the virus and for longer, said Katrina Lythgoe, an evolutionary biologist at the University of Oxford. “You’re more infectious for more days,” she said.So contagious is B.1.1.7 that Britain succeeded in driving down infections only after nearly three months of strict stay-at-home orders, plus an aggressive vaccination program. Even so, cases fell much more slowly than they did during a similar lockdown in March and April.In continental Europe, a wave of B.1.1.7 cases was building for months, mostly unnoticed beneath a steady churn of infections. The variant wave is now cresting.A shopping area in Berlin. Germany’s daily case rate has doubled, triggering a ban on nighttime gatherings in Berlin.Lena Mucha for The New York TimesA doctor vaccinated a staff worker at a hospital in Munich. Much of Europe has been overwhelmed by a more contagious version of the coronavirus called B.1.1.7.Laetitia Vancon for The New York TimesPoland’s rate of daily new cases has quintupled since mid-February, forcing the closure of most public venues. Germany’s has doubled, triggering a ban on nighttime gatherings in Berlin.In France, where B.1.1.7 is causing three-quarters of new infections, some hospitals have had to move coronavirus patients to Belgium to free up beds. Roughly as many people are dying each day from Covid-19 in Europe as were this time a year ago.For too long, government officials disregarded the threat. “Case plateaus can hide the emergence of new variants,” said Carl Pearson, a research fellow at the London School of Hygiene and Tropical Medicine. “And the higher those plateaus are, the worse the problem is.”In the United States, coronavirus infections began a rapid decline in January, soon prompting many state leaders to reopen businesses and ease restrictions. But scientists repeatedly warned that the drop would not last. After the rate bottomed out at about 55,000 cases and 1,500 deaths per day in mid-March, some states — notably Michigan — began seeing an uptick.Since then, the national numbers have steadily risen. As of Saturday, the daily count was up to nearly 69,000, and the weekly average was 19 percent higher than the figure two weeks earlier.Even when cases were falling, researchers questioned the notion that vaccinations were the reason. Millions of Americans are immunized every day, but even now only 31 percent have received a single dose of a vaccine, and just 17 percent of the population have full protection, leaving a vast majority susceptible.“The fact is that we’re still in a position now where we don’t have enough vaccinated people,” said Kristian Andersen, a virologist at the Scripps Research in San Diego. “And if we, like Texas, say we’re done with Covid-19, B.1.1.7 will come in and remind us that we are not right. I have no doubt about it.”A nurse swabbed a patient for coronavirus infection at the Desmond Tutu HIV Foundation Youth Center in Masiphumelele, near Cape Town, South Africa.Joao Silva/The New York TimesVolunteers fumigated a senior citizen center in Soweto, South Africa, in February.Joao Silva/The New York TimesThe variant is particularly pervasive in Florida, where the state lifted restrictions and initially did not see a surge. Officials in other states cited this as a rationale for reopening. But now Florida’s infection rate is curving upward.The variant may only have been obscured by what scientists like to call seasonality. Respiratory infections are usually rare in Florida in the spring, noted Sarah Cobey, an evolutionary biologist at the University of Chicago. Coronavirus infections peaked in Florida last year in the summer, as heat drove people indoors, and may do so again.“I still don’t think we’re out of the woods,” Dr. Cobey said, referring to the country at large. “If we don’t have another wave this spring, then I’m going to be really, really worried about the fall.”While most vaccines are effective against B.1.1.7, researchers are increasingly concerned about other variants that contain a mutation called E484K. (Scientists often refer to it, appropriately, as “Eek.”)This mutation has evolved independently in many variants worldwide, suggesting that it offers the virus a powerful survival advantage.In laboratory studies, the Pfizer-BioNTech and Moderna vaccines seem to be slightly less effective against B.1.351, the variant identified in South Africa. That variant contains the Eek mutation, which seems to enable the virus to partly sidestep the body’s immune response. The vaccines made by Johnson & Johnson, AstraZeneca and Novavax were even less potent against B.1.351.“I think for the next year or two, E484K will be the most concerning” mutation, said Jesse Bloom, an evolutionary biologist at the Fred Hutchinson Cancer Research Center in Seattle.The mutation slightly alters the so-called spike protein sitting on the surface of the coronavirus, making it just a bit harder for antibodies to latch on and destroy the invader.The good news is that the virus seems to have just a few survival tricks in its bag, and that makes it easier for scientists to find and block those defenses. “I’m feeling pretty good about the fact that there aren’t that many choices,” said Michel Nussenzweig, an immunologist at Rockefeller University in New York.The Eek mutation seems to be the virus’s primary defense against the immune system. Researchers in South Africa recently reported that a new vaccine directed against B.1.351 ought to fend off all other variants, as well.Pfizer, BioNTech and Moderna already are testing newly designed booster shots against B.1.351 that should work against any variants known to blunt the immune response.Instead of a new vaccine against variants, however, it may be just as effective for Americans to receive a third dose of the Pfizer-BioNtech or Moderna vaccines in six months to a year, said Dr. Anthony S. Fauci, head of the National Institute of Allergy and Infectious Diseases.That would keep antibody levels high in each recipient, overwhelming any variant — a more practical strategy than making a specialized vaccine for each new variant that emerges, he said.“My only concern about chasing all the variants is that you’d almost be playing Whac-A-Mole, you know, because they’ll keep coming up and keep coming up,” Dr. Fauci said. In one form or another, the new coronavirus is here to stay, many scientists believe. Multiple variants may be circulating in the country at the same time, as is the case for common cold coronaviruses and influenza. Keeping them at bay may require an annual shot, like the flu vaccine.The best way to deter the emergence of dangerous variants is to keep cases down now and to immunize the vast majority of the world — not just the United States — as quickly as possible. If significant pockets of the globe remain unprotected, the virus will continue to evolve in dangerous new ways.“This might be something that we have to deal with for a long time,” said Rosalind Eggo, an epidemiologist at London School of Hygiene and Tropical Medicine.Still, she added, “Even if it changes again, which it is very likely to do, we are in a better, much stronger position than a year ago to deal with it.”
Read more →