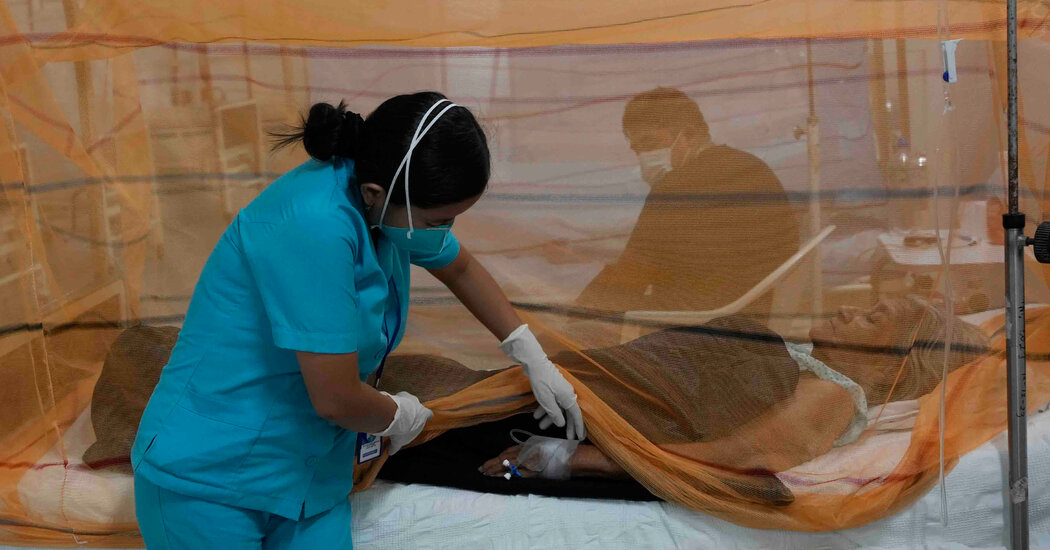
The first vaccine for malaria received major regulatory approval in 2015.It didn’t become part of vaccination programs in Africa until 2024.What if it had come faster?What if the shots had arrived9 years ago?143,000.That’s how many children’s deaths could have been averted.July 5, 2024Nurses in countries from Sierra Leone to Cameroon are packing a new vaccine into the coolers they tote to villages for immunization clinics: a shot to protect against malaria, one of the deadliest diseases for children.Babies and toddlers in eight countries in the region recently started to get the vaccine as part of their routine childhood shots. Seven other African countries are eagerly awaiting its arrival.This is a milestone in global health.But it’s also a cautionary tale about a system that is ill equipped to deliver critical tools to the people who need them most.It took decades and at least a billion dollars to reach this point. Even now, only a fraction of the children whose lives are at risk will get the vaccine this year, or next year, or the year after.It’s been clear for some time what went wrong, but almost none of those issues have been fixed. That means that the next desperately needed vaccine stands every chance of running into those same problems.Take, for example, a new vaccine for tuberculosis that started clinical trials a few months ago. If it works as well as hoped, it could save at least a million lives a year. We’ll know by 2028 if it stops tuberculosis infections. But if it follows the same trajectory, it will be at least 2038 before it’s shipped to clinics.“Children are receiving the vaccine, and for that, I am the happiest man in the world. But on the other hand, I cannot avoid being dismayed at this inexcusably long delay.”— Dr. Joe Cohen, co-inventor of the first malaria vaccineThe U.S. Army started work on a malaria vaccine back in the 1980s, hoping to protect soldiers deployed to the tropics. It teamed up with the drug company GlaxoSmithKline, and together they produced promising prototypes. But the military lost interest after a few years, and that left GSK with a problem.The people who desperately needed a malaria vaccine were in villages in sub-Saharan Africa. They would not be able to pay for a product that would cost millions of dollars to develop.GSK needed an altruistically minded partner. It found one in the nonprofit global health agency PATH, and by the late 1990s they had a vaccine to test. The Bill & Melinda Gates Foundation put up more than $200 million to test it.The clinical trials were complex, because this was a whole new type of vaccine — the first ever against a parasite — delivered to children in places with limited health systems. The process took more than a decade.Finally, in 2014, results showed this vaccine cut severe malaria cases by about a third.This was a successful result, but not as much protection as scientists had hoped to see. Still, GSK and PATH planned a production facility to make millions of doses. Gavi, the organization that procures vaccines for low- and middle-income countries, with funds from donors, would buy them.Then the Gates Foundation pulled its support.There was a shake-up in the malaria division, and the leadership reoriented toward a new goal: eliminating the disease.The new malaria team said the vaccine didn’t work well enough to justify pouring millions more dollars into it. It would be better, they said, to wait for a more effective shot in the future, and in the meantime to fund other strategies, such as genetically modifying mosquitoes.“If you go from very enthusiastic to very unenthusiastic and you’re the Gates Foundation, people pay attention.”— Dr. Robert Newman, former director, Global Malaria Program, W.H.O.The decision was driven by researchers who were looking at data. They didn’t factor in that the idea of a vaccine, even one with limited efficacy, would be so important to African parents — and African governments, which would come to see this as a classic example of a paternalistic donor ignoring their priorities. More than 300,000 children died of malaria that year.The foundation’s announcement shoved the vaccine into limbo — in ways the foundation today says it did not anticipate.“In hindsight, we could have communicated more often and more clearly about our decisions and listened more clearly to what the impact of those might have been on other institutions and their decisions.”— Dr. Chris Elias, president of global development at the Bill & Melinda Gates Foundation
.css-1ggt3fz {
padding-left: 1rem;
border-left: 0px solid var(–color-stroke-quaternary, #FFFFFF);
}
.css-7ad88g {
border-top: 1.3px solid #dc0000;
}
/* TOP AD CODE BELOW */
#top-wrapper, sponsor-wrapper { display: none; }
We are having trouble retrieving the article content.Please enable JavaScript in your browser settings.Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.Thank you for your patience while we verify access.Already a subscriber? Log in.Want all of The Times? Subscribe.
Read more →