First Sickle Cell Gene Therapy Patient, 12, Leaves Hospital
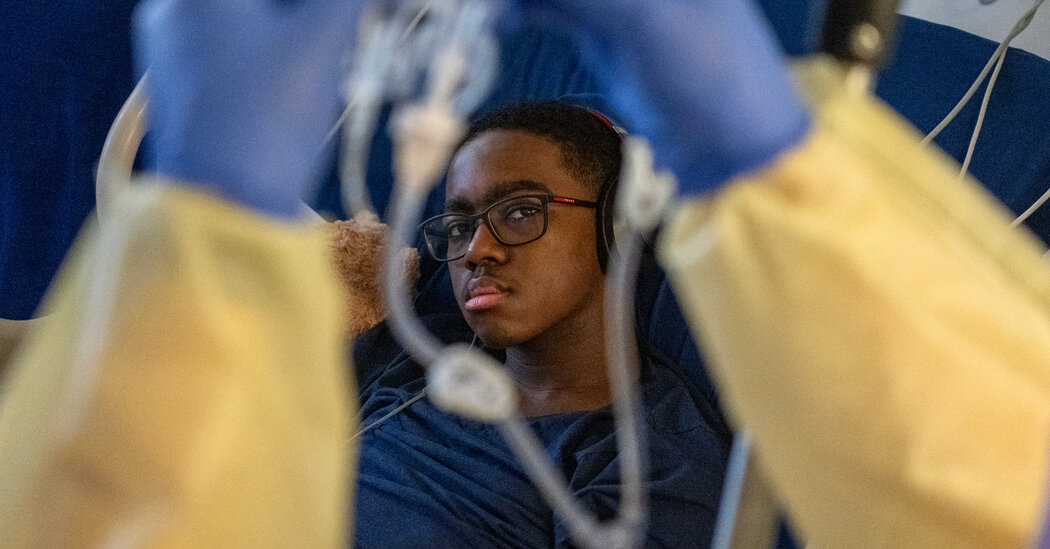
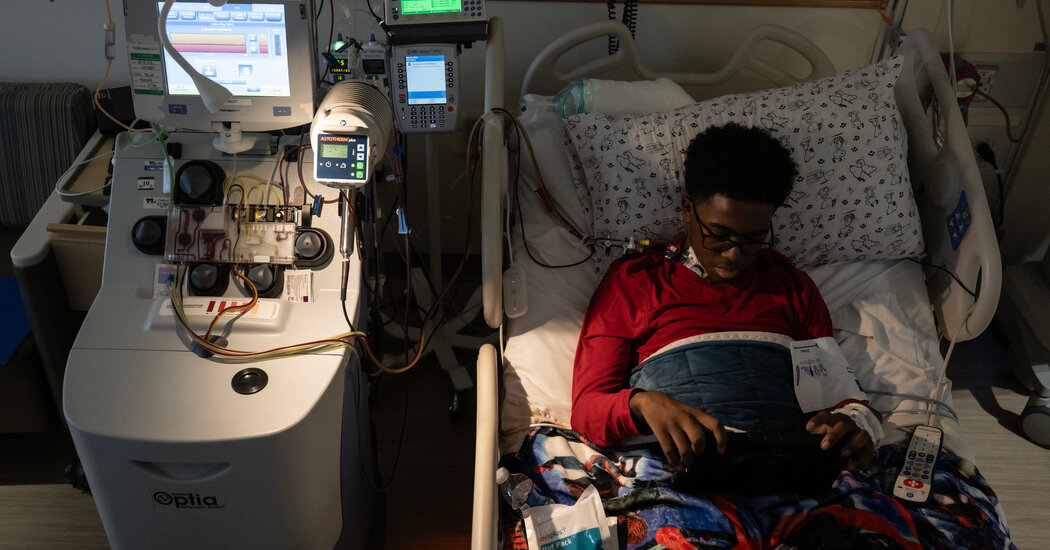
After 44 days, Kendric Cromer, 12, left the hospital. While his family feels fortunate that he was the first to receive a treatment, their difficult experiences hint at what others will be up against.Kendric Cromer, 12, left Children’s National Hospital in a wheelchair on Wednesday, wearing a T-shirt and cap printed with designs from the anime series “Naruto” and a black face mask. Staff lined the hallway, cheering and waving noisemakers. He had just become the first patient to receive a gene therapy for sickle cell since it was approved — a therapy that is expected to free him from the ravages of the disease.After 44 days in the hospital, he was a bit dazed.“I thought I would have sickle cell for the rest of my life,” he said. The disease had deprived him of his childhood, making everyday activities, like playing basketball or riding a bike, impossible because they could bring on searing pain, often resulting in hospitalizations.But despite the celebratory atmosphere, Kendric and his parents are still shuddering over what they endured during his hospital stay.Nothing, absolutely nothing — not all the discussions with doctors, not all of their reading and highlighting of texts, not the 13-page consent form that included organ damage and even death as possible outcomes — prepared them for what he would go through.About 100,000 people in the United States have sickle cell disease. For the 20,000 or so with the most severe disease, gene therapy may be their only hope of living a normal life. The disease is caused by a mutation in hemoglobin genes that leads to crescent-shaped red blood cells, which tend to get stuck in blood vessels, causing episodes of excruciating pain. The blockages can damage organs, cause strokes and shorten lives.Until recently, most saw no way out.Then, last December, the Food and Drug Administration approved a $3.1 million sickle cell gene therapy by Bluebird Bio of Somerville, Mass., and a $2.2 million treatment by Vertex Pharmaceuticals of Boston. That potentially gives patients like Kendric, if their insurance will pay for the therapy, a path to a life that is not shadowed by the ravages of the disease.We are having trouble retrieving the article content.Please enable JavaScript in your browser settings.Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times.Thank you for your patience while we verify access.Already a subscriber? Log in.Want all of The Times? Subscribe.
Read more →