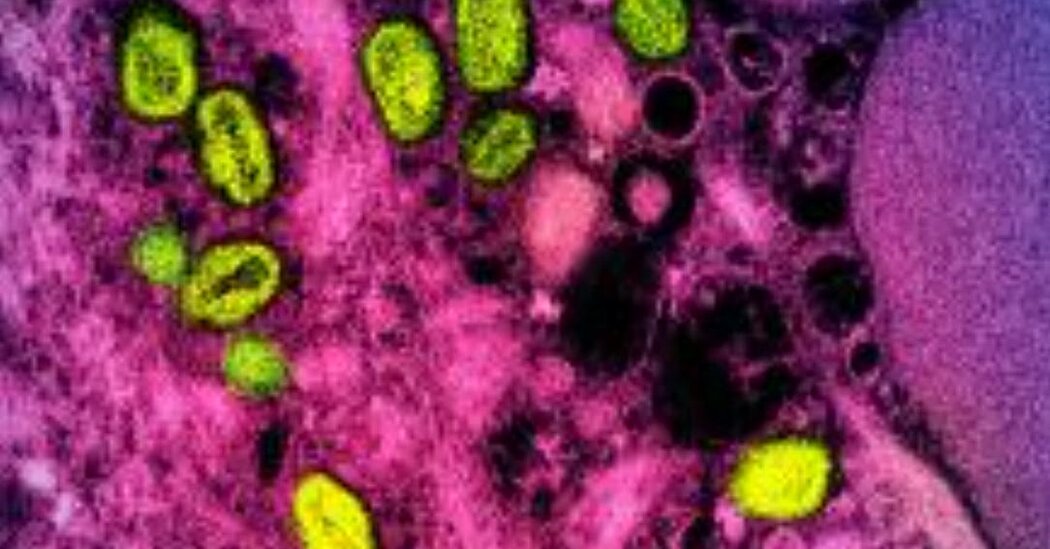
The maker of EzriCare Artificial Tears said it was recalling the eye drops after U.S. health authorities linked the product to a drug-resistant bacteria strain.The manufacturer of a brand of over-the-counter eye drops said that it was recalling the product, EzriCare Artificial Tears, after it was linked to a drug-resistant bacteria strain that has caused at least one person’s death and vision loss in five others.The Centers for Disease Control and Prevention has advised people to stop using the eye drops, as the agency investigates an outbreak of a strain of the bacteria pseudomonas aeruginosa, which can cause infections in the blood, lungs and other parts of the body. This strain of the bacteria had never been identified in the United States before the current outbreak and is resistant to a class of antibiotics called carbapenems, which are generally considered a last resort.The bacteria strain had been found in 55 people in 12 states as of Tuesday, the C.D.C. said. The agency said that the infections had caused one death, vision loss in five of 11 people who had eye infections, and some hospitalizations.Global Pharma, the Indian company that manufactures the EzriCare eye drops, said that it was recalling the eye drops “out of an abundance of caution.”“Global Pharma is fully cooperating with U.S. federal authorities, and is continuing to investigate this matter, but thus far we have not determined whether our manufacturing facility is the source of the contamination,” the company said in an emailed statement.Most of the people affected by the outbreak used artificial tears before the infections, the C.D.C. said. They had reported using more than 10 brands of artificial tears, and some patients used more than one, but EzriCare Artificial Tears is the most common brand, the agency said.The C.D.C. said that it had found the drug-resistant bacteria strain in opened bottles of the EzriCare eye drops collected from patients with and without eye infections. The agency is testing unopened bottles to determine if contamination occurred during the manufacturing process.The bacteria strain was found in people in California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington and Wisconsin between May 2022 and January, according to the C.D.C. Of those 55 cases, 35 were linked to four health-care facility clusters, the agency said.The C.D.C. said that people who have used EzriCare Artificial Tears and who have signs of an eye infection should seek medical care immediately. The symptoms can include yellow, green or clear discharge from the eye, redness of the eye or eyelid, increased sensitivity to light and eye pain or discomfort.The manufacturer of EzriCare Artificial Tears said it was recalling the product out of “an abundance of caution.” EzriCareDr. Thomas L. Steinemann, a spokesman for the American Academy of Ophthalmology, said that people did not need to be “too terribly concerned” about using other types of eye drops.“We use them for tears, we use them for antibiotics, we use them to treat glaucoma.” Dr. Steinemann said. “We use eye drop bottles every day, and I think for the vast majority of users of eye drop bottles there’s no cause for alarm.”Dr. Steinemann, an ophthalmologist at MetroHealth Medical Center in Cleveland, noted that the C.D.C. report said the EzriCare artificial tears were preservative-free, which means that, if contaminated, they do not have anything to prevent the growth of bacteria.He said that doctors often recommend preservative-free artificial tears to patients if they are using them more than four times a day because preservatives can worsen eye irritation. He said that he had only ever heard of preservative-free eye drops that were available in single-use vials that cannot be closed and used again later.“That to me stuck out when I read the C.D.C. report is that, at least for EzriCare, these products are dispensed in what we call multidose bottles, meaning that people are reusing the bottle,” Dr. Steinemann said. “But the bottle doesn’t have any preservatives, which I think might set the stage for either contamination or bacterial overgrowth in the bottle.”When people use any type of eye drops, Dr. Steinemann said, they should wash their hands, close the bottle after using it and not touch the tip, because that would risk making the drops not sterile. “Don’t touch the bottle to your eye or to your face or to your nose,” he said.EzriCare, a drug company based in New Jersey, said in a statement on Wednesday that it did not manufacture the eye drops and was involved only in designing the product’s label and in marketing it to customers.EzriCare said that it was first told about the C.D.C. investigation on Jan. 20 and “immediately took action to stop any further distribution or sale of EzriCare Artificial Tears.” The company said it had also been trying to contact customers to tell them to stop using the eye drops.Public health officials have warned that more must be done globally to stem the spread of drug-resistant infections, which occur when bacteria and fungi evolve to outsmart the antibiotic and antifungal drugs that have been developed to destroy them. The more antibiotics and antifungal medicines are given to people and livestock, the more likely resistance will occur, health officials have said.Nearly 30,000 people in the United States died in 2020 from drug-resistant infections, according to an analysis by the C.D.C., 15 percent more than in 2019. The increase was driven mostly by the coronavirus, which in the early days of the pandemic was a mystery to medical professionals. Many turned to antibiotics to try to treat the illness before vaccines and other treatments were available.Each year, more than 700,000 people across the world die from drug-resistant infections. The United Nations warned in 2019 that, without concerted action, these infections could kill 10 million people annually by 2050 and trigger a global economic crisis.
Read more →